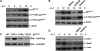IL-7 promotes T cell proliferation through destabilization of p27Kip1 - PubMed (original) (raw)
IL-7 promotes T cell proliferation through destabilization of p27Kip1
Wen Qing Li et al. J Exp Med. 2006.
Abstract
Interleukin (IL)-7 is required for survival and homeostatic proliferation of T lymphocytes. The survival effect of IL-7 is primarily through regulation of Bcl-2 family members; however, the proliferative mechanism is unclear. It has not been determined whether the IL-7 receptor actually delivers a proliferative signal or whether, by promoting survival, proliferation results from signals other than the IL-7 receptor. We show that in an IL-7-dependent T cell line, cells protected from apoptosis nevertheless underwent cell cycle arrest after IL-7 withdrawal. This arrest was accompanied by up-regulation of the cyclin-dependent kinase inhibitor p27Kip1 through a posttranslational mechanism. Overexpression of p27Kip1 induced G1 arrest in the presence of IL-7, whereas knockdown of p27Kip1 by small interfering RNA promoted S phase entry after IL-7 withdrawal. CD4 or CD8 T cells transferred into IL-7-deficient hosts underwent G1 arrest, whereas 27Kip1-deficient T cells underwent proliferation. We observed that IL-7 withdrawal activated protein kinase C (PKC)theta and that inhibition of PKCtheta with a pharmacological inhibitor completely blocked the rise of p27Kip1 and rescued cells from G1 arrest. The conventional pathway to breakdown of p27Kip1 is mediated by S phase kinase-associated protein 2; however, our evidence suggests that PKCtheta acts via a distinct, unknown pathway inducing G1 arrest after IL-7 withdrawal from T cells. Hence, IL-7 maintains T cell proliferation through a novel pathway of p27Kip1 regulation.
Figures
Figure 1.
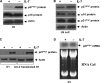
Proliferative effect of IL-7 is independent of survival effect. D1 or _bcl-2_–transfected D1 cells were cultured in the presence or absence of IL-7 for different time points. Cell cycle and survival were analyzed by PI staining after flow cytometric assay. G1 peak, S phase, G2/M phase, and apoptotic populations are indicated. Ap, apoptosis. Percentage of cells in different populations was calculated by ModFit LT software. The results are representative of three independent experiments.
Figure 2.
IL-7 down-regulates p27Kip1 posttranslationally. (A) D1 cells were cultured with (+) or without (−) IL-7 for 12 h. Cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies against p27Kip1, p16, and β-actin. (B) LN T cells were isolated and grown with or without IL-7 for 24 h. Protein levels were measured by immunoblotting using antibodies against p27Kip1, p16, p21Cip1, and β-actin. (C) Cell lysates were prepared from D1 or _bcl-2_–transfected D1 cells cultured with or without IL-7 for 12 h. p27Kip1 protein level was measured by immunoblotting. (D) D1 cells were cultured with (+) or without (−) IL-7 for 12 h. Total RNAs were isolated and 20 μg total RNA was loaded to agarose gel (bottom). The RNA blot was probed with 32P-labeled p27Kip1 cDNA fragment (top).
Figure 3.
p27Kip1 causes G1 arrest without effect on survival in D1 cells. D1 cells were transfected with p27Kip1 and cultured with IL-7 for certain time points. Cell cycle and survival were analyzed by PI staining after flow cytometric analysis. G1 peak, S phase, G2/M phase, and apoptotic populations are indicated. Ap, apoptosis. Percentage of cells in different populations was calculated by ModFit LT software. The results are representative of three independent experiments.
Figure 4.
siRNA knockdown of p27Kip1 expression protects from cell cycle arrest induced by IL-7 deprivation in vitro. D1 cells transfected with either pMIG-hU6-sip27Kip1 (sip27Kip1, small interfering p27Kip1) or pMIG-hU6-NC (Control, scrambled siRNA control) were kept in IL-7 or without IL-7 for 15 h. Total cell extracts were subjected to immunoblotting with antibodies against p27Kip1 and β-actin (top). Cell cycles were determined by PI staining (bottom). Percentage of cells in different populations was calculated by ModFit LT software. A representative experiment of three performed is shown.
Figure 5.
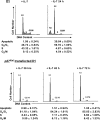
The loss of p27Kip1 compensates the loss of IL-7–promoting T cell proliferation in vivo. Lymphocytes were isolated from LNs from p27−/− or WT mice and labeled by CFSE, and then transferred intravenously into irradiated IL-7−/−/Rag−/− or Rag−/− mice. 6 d later, spleens and LNs were removed from recipient mice and lymphocytes were prepared. Donor T lymphocytes were analyzed for CFSE intensity by gating on CD4+ and CD8+ cells using flow cytometry. Numbers over the peaks indicate the number of cell divisions. Vertical line represents the gate used for calculating the percentage of cells that had divided, which was calculated using WinMDI software (reference 3). Data are representative of two experiments.
Figure 6.
Inhibition of PKC after IL-7 withdrawal blocks p27Kip1 induction through a Skp2 and T187 phosphorylation–independent pathway. (A) Protein levels of p27Kip1 and phospho-p27Kip1 were measured in D1 cells. Cells were deprived of IL-7 for various time points and cell lysates were resolved by SDS-PAGE. Immunoblotting was performed with antibodies against p27Kip1, phospho-p27Kip1 (T187 or S10), and β-actin. (B) The effect of PKC inhibitor on p27Kip1 stability was determined. IL-7 was withdrawn from D1 cells for 12 h and 5 μM of the PKC inhibitor Gö6850 was added at the time of IL-7 withdrawal. Protein levels were measured by immunoblotting using antibodies against p27Kip1, phospho-p27Kip1 (T187), and β-actin. 0.1% of DMSO was included as a negative control. (C) p27Kip1 stability affected by phosphorylation was analyzed in D1 cells overexpressing the FLAG-tagged p27Kip1 WT, single mutant T187A, S10A, or T187D, respectively. Extrogenous protein levels were detected by immunoblotting using antibody against FLAG. The GFP expression level was also measured as transfection efficiency control. (D) Skp2 and Cks1 expressions were measured in D1 cells. Cell lysates were prepared from cells deprived of IL-7 for 4 and 12 h, or cells cultured without IL-7 for 12 h and 5 μM of the PKC inhibitor Gö6850. Immunoblotting was analyzed by specific antibodies against Skp2, Cks1, and β-actin. 0.1% of DMSO was included as a negative control.
Figure 7.
PKCθ is activated and inhibition of PKCθ prevents cell cycle G1 arrest after IL-7 withdrawal. (A) PKC activation was analyzed in D1 cells of IL-7 withdrawal for 12 h. Cell lysates were resolved by SDS-PAGE and immunoblotted using a phospho-PKC sample kit. Total PKCθ was measured as a loading control. (B) PKCθ activation was determined in D1 cells after IL-7 deprivation. Cell lysates were made from cells deprived of IL-7 for 4, 8, and 12 h, and PKCθ activation was assayed by immunoblotting using an antibody specific for PKCθ phosphorylation at threonine 538. (C) D1 cells were cultured without IL-7 and treated with 5 μM Gö6850 for 15 h. Cell cycle was analyzed by PI staining and the percentage of cells in different populations was calculated by ModFit LT software. Results were pooled from three experiments.
Figure 8.
Model: IL-7 regulation of p27Kip1 degradation in T cells. Top: In the presence of IL-7, the major pathway to p27Kip1 degradation is shown on the left and involves an unknown ubiquitin E3 ligase complex, possibly KPC1 and KPC2. The minor pathway on the right is the conventional Skp1/Cks1 pathway and depends on phosphorylation of T187. Bottom: In the absence of IL-7, PKCθ is activated, inactivating the major p27Kip1 degradation pathway and resulting in p27Kip1 accumulation. The minor pathway of degradation is inactivated later and also contributes to p27Kip1 accumulation.
Similar articles
- Role of proteasomes in T cell activation and proliferation.
Wang X, Luo H, Chen H, Duguid W, Wu J. Wang X, et al. J Immunol. 1998 Jan 15;160(2):788-801. J Immunol. 1998. PMID: 9551914 - P27kip1 regulates the cell cycle arrest and survival of activated T lymphocytes in response to interleukin-2 withdrawal.
Huleatt JW, Cresswell J, Bottomly K, Crispe IN. Huleatt JW, et al. Immunology. 2003 Apr;108(4):493-501. doi: 10.1046/j.1365-2567.2003.01605.x. Immunology. 2003. PMID: 12667211 Free PMC article. - Protein kinase C-theta-mediated signals enhance CD4+ T cell survival by up-regulating Bcl-xL.
Manicassamy S, Gupta S, Huang Z, Sun Z. Manicassamy S, et al. J Immunol. 2006 Jun 1;176(11):6709-16. doi: 10.4049/jimmunol.176.11.6709. J Immunol. 2006. PMID: 16709830 - Limiting amounts of p27Kip1 correlates with constitutive activation of cyclin E-CDK2 complex in HTLV-I-transformed T-cells.
Cereseto A, Washington Parks R, Rivadeneira E, Franchini G. Cereseto A, et al. Oncogene. 1999 Apr 15;18(15):2441-50. doi: 10.1038/sj.onc.1202567. Oncogene. 1999. PMID: 10229195
Cited by
- T-cell intrinsic and extrinsic mechanisms of p27Kip1 in the regulation of CD8 T-cell memory.
Jatzek A, Marie Tejera M, Plisch EH, Fero ML, Suresh M. Jatzek A, et al. Immunol Cell Biol. 2013 Feb;91(2):120-9. doi: 10.1038/icb.2012.71. Epub 2012 Dec 4. Immunol Cell Biol. 2013. PMID: 23207280 Free PMC article. - Interleukin-7 Modulates Anti-Tumor CD8+ T Cell Responses via Its Action on Host Cells.
Deiser K, Stoycheva D, Bank U, Blankenstein T, Schüler T. Deiser K, et al. PLoS One. 2016 Jul 22;11(7):e0159690. doi: 10.1371/journal.pone.0159690. eCollection 2016. PLoS One. 2016. PMID: 27447484 Free PMC article. - Adaptive immunity to murine skin commensals.
Shen W, Li W, Hixon JA, Bouladoux N, Belkaid Y, Dzutzev A, Durum SK. Shen W, et al. Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):E2977-86. doi: 10.1073/pnas.1401820111. Epub 2014 Jul 7. Proc Natl Acad Sci U S A. 2014. PMID: 25002505 Free PMC article. - An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance.
Ouyang W, Beckett O, Flavell RA, Li MO. Ouyang W, et al. Immunity. 2009 Mar 20;30(3):358-71. doi: 10.1016/j.immuni.2009.02.003. Epub 2009 Mar 12. Immunity. 2009. PMID: 19285438 Free PMC article. - Pituitary adenylate cyclase-activating peptide and vasoactive intestinal polypeptide bias Langerhans cell Ag presentation toward Th17 cells.
Ding W, Manni M, Stohl LL, Zhou XK, Wagner JA, Granstein RD. Ding W, et al. Eur J Immunol. 2012 Apr;42(4):901-11. doi: 10.1002/eji.201141958. Eur J Immunol. 2012. PMID: 22531916 Free PMC article.
References
- Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. - PubMed
- Seddon, B., P. Tomlinson, and R. Zamoyska. 2003. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 4:680–686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials