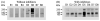Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery - PubMed (original) (raw)
Comparative Study
Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery
H Zhou et al. Kidney Int. 2006 Apr.
Abstract
Urinary exosomes containing apical membrane and intracellular fluid are normally secreted into the urine from all nephron segments, and may carry protein markers of renal dysfunction and structural injury. We studied methods for collection, storage, and preservation of urinary exosomal proteins. We collected urine from healthy volunteers, added protease inhibitors, and stored urine samples at 4, -20, and -80 degrees C for 1 week or 7 months. Samples were thawed with and without extensive vortexing, and three fractions were isolated: urinary sediment, supernatant, and exosome fraction. Protein concentration, electrophoresis patterns, and abundance of seven exosome-associated proteins were measured. Exosome-associated proteins were not detected in sediment or supernatant fractions. Protease inhibitors prevented degradation of exosome-associated proteins. Freezing at -20 degrees C caused a major loss in exosomes compared to fresh urine. In contrast, recovery after freezing at -80 degrees C was almost complete. Extensive vortexing after thawing markedly increased exosome recovery in urine frozen at -20 or -80 degrees C, even if frozen for 7 months. The recovery from first and second morning urine was similar. The abundance of cytosolic exosome-associated proteins did not decrease during long-term storage. We concluded: (1) protease inhibitors are essential for preservation; (2) storage at -80 degrees C with extensive vortexing after thawing maximizes the recovery of urinary exosomes; (3) the difference between first and second morning urine exosome-associated protein was small, suggesting minimal protein degradation in the urinary tract/bladder; (4) urinary exosomes remain intact during long-term storage. These urine collection, storage, and processing conditions may be useful for future biomarker discovery efforts.
Figures
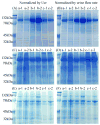
Figure 1. The isolation of three urinary fractions
Urinary sediment (17,000 × g pellet), exosome fraction (200,000 × g pellet) and acetone insoluble supernatant (17,000 × g supernatant precipitated by acetone) were isolated as described in methods.
Figure 2. The effect of protease inhibitors
Eight fresh urine samples were collected without (A) and with (B) protease inhibitors, and exosome fractions were prepared, and evaluated by western blotting for NKCC2. Sample loading was normalized by urine creatinine. B1-B8 and D1-D8 represent 2 sets of different volunteers.
Figure 3. The effect of storage and vortexing
Samples were pooled from 3 individuals. Amount of urinary exosome-associated protein (200,000 × g pellet) (A), Coomassie blue-stained gel of equal fraction volume of protein (B), Western blot (10 :l aliquot) for NHE3, TSG101, ALIX and AQP2 (C).
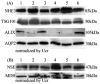
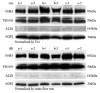
Figure 4. Effect of long-term storage
(A) Western blot of NHE3, TSG101, ALIX and AQP2 abundance in exosome fraction normalized by urine creatinine from 10 ml freshly-collected urine samples (lane 1-3) or after long-term storage (-80°C for 7 months; lane 4-6) from 3 different individuals. Lane 1 and 4, lane 2 and 5, lane 3 and 6 are from the same volunteer. (B) Western blot of cytosolic exosome-associated proteins (NSE and MDH) normalized by urine creatinine in fresh (lane 1-3) and long-term stored (-80°C for 7 months; lane 4-6) urine samples from 3 different individuals. Lanes as in A.
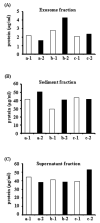
Figure 5. The amount of total protein in three different urinary fractions of human first and second morning urine
The amount of total protein in first (open bar) and second (closed bar) morning urine samples in different urinary fractions from three volunteers (a, b and c), 1: morning first urine, 2: morning second urine. Protein content in the exosome fraction (after 200,000 × g 1hr spin, A), urinary sediment proteins (after 17,000 × g 15 min spin, B), and supernatant proteins (after 17,000 × g 15 min spin, C).
Figure 6. Protein electrophoretic patterns of three different urinary fractions of human first and second morning urine
Coomassie blue-stained gels of urinary proteins in exosome fraction after 200,000 × g 1hr spin (A and B), urinary sediment proteins after 17,000 × g 15 min spin (C and D) and supernatant proteins after 17,000 × g 15 min spin (E and F) normalized by urinary creatinine (A, C, E) or urine flow rate (B, D, F). Legend: a, b and c represent different volunteers, 1: first morning urine, 2: second morning urine.
Figure 7. Specific exosome-associated proteins in first and second morning urine
Abundance of NHE3, TSG101, ALIX and AQP2 by western blotting in the urinary exosome fraction normalized by urinary creatinine (A) or urine flow rate (B). Legend: a, b and c represent different volunteers, 1: first morning urine, 2: second morning urine.
Similar articles
- Effect of long-term storage of urine samples on measurement of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL).
van de Vrie M, Deegens JK, van der Vlag J, Hilbrands LB. van de Vrie M, et al. Am J Kidney Dis. 2014 Apr;63(4):573-6. doi: 10.1053/j.ajkd.2013.10.010. Epub 2013 Nov 21. Am J Kidney Dis. 2014. PMID: 24268306 - Storage of plasma-derived exosomes: evaluation of anticoagulant use and preserving temperatures.
Yang C, Han J, Liu H, He Y, Zhang Z, Liu X, Waqas F, Zhang L, Duan H, He J, Dong L. Yang C, et al. Platelets. 2024 Dec;35(1):2337255. doi: 10.1080/09537104.2024.2337255. Epub 2024 Apr 17. Platelets. 2024. PMID: 38630028 - Identification and proteomic profiling of exosomes in human urine.
Pisitkun T, Shen RF, Knepper MA. Pisitkun T, et al. Proc Natl Acad Sci U S A. 2004 Sep 7;101(36):13368-73. doi: 10.1073/pnas.0403453101. Epub 2004 Aug 23. Proc Natl Acad Sci U S A. 2004. PMID: 15326289 Free PMC article. - Urine Exosomes: An Emerging Trove of Biomarkers.
Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PS. Street JM, et al. Adv Clin Chem. 2017;78:103-122. doi: 10.1016/bs.acc.2016.07.003. Epub 2016 Aug 18. Adv Clin Chem. 2017. PMID: 28057185 Review. - Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signalling.
Dear JW, Street JM, Bailey MA. Dear JW, et al. Proteomics. 2013 May;13(10-11):1572-80. doi: 10.1002/pmic.201200285. Epub 2013 Feb 15. Proteomics. 2013. PMID: 23129434 Review.
Cited by
- The nephroprotective action of Passiflora edulis in streptozotocin-induced diabetes.
Araújo Galdino O, de Souza Gomes I, Ferreira de Almeida Júnior R, Conceição Ferreira de Carvalho MI, Abreu BJ, Abbott Galvão Ururahy M, Cabral B, Zucolotto Langassner SM, Costa de Souza KS, Augusto de Rezende A. Araújo Galdino O, et al. Sci Rep. 2022 Oct 20;12(1):17546. doi: 10.1038/s41598-022-21826-9. Sci Rep. 2022. PMID: 36266308 Free PMC article. - Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers.
Wood SL, Knowles MA, Thompson D, Selby PJ, Banks RE. Wood SL, et al. Nat Rev Urol. 2013 Apr;10(4):206-18. doi: 10.1038/nrurol.2013.24. Epub 2013 Feb 26. Nat Rev Urol. 2013. PMID: 23443013 Review. - The present and future of prostate cancer urine biomarkers.
Rigau M, Olivan M, Garcia M, Sequeiros T, Montes M, Colás E, Llauradó M, Planas J, Torres Id, Morote J, Cooper C, Reventós J, Clark J, Doll A. Rigau M, et al. Int J Mol Sci. 2013 Jun 17;14(6):12620-49. doi: 10.3390/ijms140612620. Int J Mol Sci. 2013. PMID: 23774836 Free PMC article. Review. - Extracellular vesicles in immunomodulation and tumor progression.
Marar C, Starich B, Wirtz D. Marar C, et al. Nat Immunol. 2021 May;22(5):560-570. doi: 10.1038/s41590-021-00899-0. Epub 2021 Mar 22. Nat Immunol. 2021. PMID: 33753940 Free PMC article. Review. - An in-depth comparison of the male pediatric and adult urinary proteomes.
Froehlich JW, Vaezzadeh AR, Kirchner M, Briscoe AC, Hofmann O, Hide W, Steen H, Lee RS. Froehlich JW, et al. Biochim Biophys Acta. 2014 May;1844(5):1044-50. doi: 10.1016/j.bbapap.2013.05.008. Epub 2013 May 22. Biochim Biophys Acta. 2014. PMID: 23707565 Free PMC article.
References
- Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–89. - PubMed
- du Cheyron D, Daubin C, Poggioli J, et al. Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am J Kidney Dis. 2003;42:497–506. - PubMed
- Hoorn EJ, Pisitkun T, Zietse R, et al. Prospects for urinary proteomics: Exosomes as a source of urinary biomarkers. Nephrology (Carlton) 2005;10:283–90. - PubMed
- Thongboonkerd V, Klein E, Klein JB. Sample preparation for 2-D proteomic analysis. Contrib Nephrol. 2004;141:11–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous