Generation and characterization of telomere length maintenance in tankyrase 2-deficient mice - PubMed (original) (raw)
Generation and characterization of telomere length maintenance in tankyrase 2-deficient mice
Y Jeffrey Chiang et al. Mol Cell Biol. 2006 Mar.
Abstract
Telomere length and function are crucial factors that determine the capacity for cell proliferation and survival, mediate cellular senescence, and play a role in malignant transformation in eukaryotic systems. The telomere length of a specific mammalian species is maintained within a given range by the action of telomerase and telomere-associated proteins. TRF1 is a telomere-associated protein that inhibits telomere elongation by its binding to telomere repeats, preventing access to telomerase. Human TRF1 interacts with tankyrase 1 and tankyrase 2 proteins, two related members of the tankyrase family shown to have poly(ADP-ribose) polymerase activity. Human tankyrase 1 is reported to ADP-ribosylate TRF1 and to down-regulate the telomeric repeat binding activity of TRF1, resulting in telomerase-dependent telomere elongation. Human tankyrase 2 is proposed to have activity similar to that of tankyrase 1, although tankyrase 2 function has been less extensively characterized. In the present study, we have assessed the in vivo function of mouse tankyrase 2 by germ line gene inactivation and show that inactivation of tankyrase 2 does not result in detectable alteration in telomere length when monitored through multiple generations of breeding. This finding suggests that either mouse tankyrases 1 and 2 have redundant functions in telomere length maintenance or that mouse tankyrase 2 differs from human tankyrase 2 in its role in telomere length maintenance. Tankyrase 2 deficiency did result in a significant decrease in body weight sustained through at least the first year of life, most marked in male mice, suggesting that tankyrase 2 functions in potentially telomerase-independent pathways to affect overall development and/or metabolism.
Figures
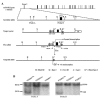
FIG.1.
Generation of TANK2-deficient mice by gene targeting. (A) Gene targeting strategy and restriction map of TANK2 gene. Filled boxes indicate exons, and labeled boxes indicate neomycin (neo) resistance or herpesvirus thymidine kinase (tk) genes. loxP sites are indicated. (B) Southern blot analysis of ES cell DNA. The 8.5-kb band represents the germ line allele. The 5.4- and 4-kb bands represent the targeted alleles. (C) PCR analysis for the conditional TANK2 knockout mouse genotype. The 270- and 340-bp PCR products represent the wild-type and floxed alleles, respectively. (D) PCR analysis for the constitutive TANK2 knockout mouse genotype. The 270- and 240-bp PCR products represent the wild-type and knockout alleles, respectively. (E) RT-PCR analysis for TANK2 mRNA expression. 5′-TANK2 and 3′-TANK2 PCR products represent upstream and downstream cDNA of TANK2, respectively. Actin cDNA was the RT-PCR loading control.
FIG.1.
Generation of TANK2-deficient mice by gene targeting. (A) Gene targeting strategy and restriction map of TANK2 gene. Filled boxes indicate exons, and labeled boxes indicate neomycin (neo) resistance or herpesvirus thymidine kinase (tk) genes. loxP sites are indicated. (B) Southern blot analysis of ES cell DNA. The 8.5-kb band represents the germ line allele. The 5.4- and 4-kb bands represent the targeted alleles. (C) PCR analysis for the conditional TANK2 knockout mouse genotype. The 270- and 340-bp PCR products represent the wild-type and floxed alleles, respectively. (D) PCR analysis for the constitutive TANK2 knockout mouse genotype. The 270- and 240-bp PCR products represent the wild-type and knockout alleles, respectively. (E) RT-PCR analysis for TANK2 mRNA expression. 5′-TANK2 and 3′-TANK2 PCR products represent upstream and downstream cDNA of TANK2, respectively. Actin cDNA was the RT-PCR loading control.
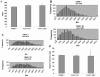
FIG. 2.
Telomere length was not altered in TANK2 knockout mice. (A) Spleen cells were isolated from TANK2+/+ (n = 7), TANK2−/−G1 (n = 7), and TANK2−/−G5/G7 (n = 7) mice, and relative telomere length was determined by Flow-FISH. The fluorescein isothiocyanate fluorescent signal of the cell binding telomeric probe was converted to arbitrary units of molecule equivalents of soluble fluorescence (MESF). (B and C) Histograms, based on Q-FISH data from the comparison of one TANK2+/+ mouse with one TANK2−/−G1 mouse (B) and the comparison of one TANK2+/+ mouse with one TANK2−/−G7 mouse (C), depict the distribution of fluorescent intensities corresponding to individual telomeres of different lengths present in metaphase chromosomes. (D) Spleen cells were isolated from TANK2+/+ (n = 8), TANK2−/−G1 (n = 8), and TANK2−/−G5/G7 (n = 9) mice, and relative telomere length was determined by Q-FISH. The average of fluorescent intensities from each mouse was normalized to that of a TANK2+/+ mouse (defined as 100). The relative telomere length of each strain of mice is plotted.
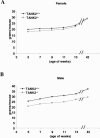
FIG. 3.
The body weights of tankyrase 2 knockout mice were reduced. (A) Body weight changes in female TANK+/+ (n = 5) and TANK−/− (n = 6) mice. (B) Body weight changes in male TANK+/+ (n = 6) and TANK−/− (n = 7) mice.
Similar articles
- Tankyrase promotes telomere elongation in human cells.
Smith S, de Lange T. Smith S, et al. Curr Biol. 2000 Oct 19;10(20):1299-302. doi: 10.1016/s0960-9822(00)00752-1. Curr Biol. 2000. PMID: 11069113 - Telomere elongation by a mutant tankyrase 1 without TRF1 poly(ADP-ribosyl)ation.
Muramatsu Y, Tahara H, Ono T, Tsuruo T, Seimiya H. Muramatsu Y, et al. Exp Cell Res. 2008 Mar 10;314(5):1115-24. doi: 10.1016/j.yexcr.2007.12.005. Epub 2007 Dec 14. Exp Cell Res. 2008. PMID: 18221737 - Cross-species difference in telomeric function of tankyrase 1.
Muramatsu Y, Ohishi T, Sakamoto M, Tsuruo T, Seimiya H. Muramatsu Y, et al. Cancer Sci. 2007 Jun;98(6):850-7. doi: 10.1111/j.1349-7006.2007.00462.x. Epub 2007 Apr 13. Cancer Sci. 2007. PMID: 17433040 Free PMC article. - The telomeric PARP, tankyrases, as targets for cancer therapy.
Seimiya H. Seimiya H. Br J Cancer. 2006 Feb 13;94(3):341-5. doi: 10.1038/sj.bjc.6602951. Br J Cancer. 2006. PMID: 16421589 Free PMC article. Review. - Tankyrase function at telomeres, spindle poles, and beyond.
Hsiao SJ, Smith S. Hsiao SJ, et al. Biochimie. 2008 Jan;90(1):83-92. doi: 10.1016/j.biochi.2007.07.012. Epub 2007 Jul 24. Biochimie. 2008. PMID: 17825467 Review.
Cited by
- The Axin/TNKS complex interacts with KIF3A and is required for insulin-stimulated GLUT4 translocation.
Guo HL, Zhang C, Liu Q, Li Q, Lian G, Wu D, Li X, Zhang W, Shen Y, Ye Z, Lin SY, Lin SC. Guo HL, et al. Cell Res. 2012 Aug;22(8):1246-57. doi: 10.1038/cr.2012.52. Epub 2012 Apr 3. Cell Res. 2012. PMID: 22473005 Free PMC article. - Analysis of poly(ADP-Ribose) polymerases in Arabidopsis telomere biology.
Boltz KA, Jasti M, Townley JM, Shippen DE. Boltz KA, et al. PLoS One. 2014 Feb 13;9(2):e88872. doi: 10.1371/journal.pone.0088872. eCollection 2014. PLoS One. 2014. PMID: 24551184 Free PMC article. - TRF1 controls telomere length and mitotic fidelity in epithelial homeostasis.
Muñoz P, Blanco R, de Carcer G, Schoeftner S, Benetti R, Flores JM, Malumbres M, Blasco MA. Muñoz P, et al. Mol Cell Biol. 2009 Mar;29(6):1608-25. doi: 10.1128/MCB.01339-08. Epub 2009 Jan 5. Mol Cell Biol. 2009. PMID: 19124610 Free PMC article. - Whole proteome analysis of human tankyrase knockout cells reveals targets of tankyrase-mediated degradation.
Bhardwaj A, Yang Y, Ueberheide B, Smith S. Bhardwaj A, et al. Nat Commun. 2017 Dec 20;8(1):2214. doi: 10.1038/s41467-017-02363-w. Nat Commun. 2017. PMID: 29263426 Free PMC article. - Telomere dynamics: the means to an end.
Matulić M, Sopta M, Rubelj I. Matulić M, et al. Cell Prolif. 2007 Aug;40(4):462-74. doi: 10.1111/j.1365-2184.2007.00452.x. Cell Prolif. 2007. PMID: 17635515 Free PMC article. Review.
References
- Baerlocher, G. M., J. Mak, T. Tien, and P. M. Lansdorp. 2002. Telomere length measurement by fluorescence in situ hybridization and flow cytometry: tips and pitfalls. Cytometry 47:89-99. - PubMed
- Baumann, P., and T. R. Cech. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292:1171-1175. - PubMed
- Blackburn, E. H. 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579:859-862. - PubMed
- Blasco, M. A., H. W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials