Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant metastatic pancreatic cancer - PubMed (original) (raw)
Clinical Trial
. 2006 Mar 27;94(6):785-91.
doi: 10.1038/sj.bjc.6603026.
L Pasetto, G Aprile, S Cordio, E Bonetto, S Dell'Oro, P Passoni, L Piemonti, C Fugazza, G Luppi, C Milandri, R Nicoletti, A Zerbi, G Balzano, V Di Carlo, A A Brandes
Affiliations
- PMID: 16508631
- PMCID: PMC2361378
- DOI: 10.1038/sj.bjc.6603026
Clinical Trial
Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant metastatic pancreatic cancer
M Reni et al. Br J Cancer. 2006.
Abstract
Limited information on salvage treatment in patients affected by pancreatic cancer is available. At failure, about half of the patients present good performance status (PS) and are candidate for further treatment. Patients >18 years, PS >or=50, with metastatic pancreatic adenocarcinoma previously treated with gemcitabine-containing chemotherapy, and progression-free survival (PFS) <12 months received a combination of raltitrexed (3 mg m(-2)) and oxaliplatin (130 mg m(-2)) every 3 weeks until progression, toxicity, or a maximum of six cycles. A total of 41 patients received 137 cycles of chemotherapy. Dose intensity for both drugs was 92% of the intended dose. Main grade >2 toxicity was: neutropenia in five patients (12%), thrombocytopenia, liver and vomiting in three (7%), fatigue in two (5%). In total, 10 patients (24%) yielded a partial response, 11 a stable disease. Progression-free survival at 6 months was 14.6%. Median survival was 5.2 months. Survival was significantly longer in patients with previous PFS >6 months and in patients without pancreatic localisation. A clinically relevant improvement of quality of life was observed in numerous domains. Raltitrexed-oxaliplatin regimen may constitute a treatment opportunity in gemcitabine-resistant metastatic pancreatic cancer. Previous PFS interval may allow the identification of patients who are more likely to benefit from salvage treatment.
Figures
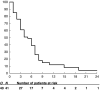
Figure 1
Overall survival. _N_=number of eligible patients. _O_=total number of events at the final analysis. Subsequent numbers are the number of patients at risk.
Similar articles
- Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study.
Tsavaris N, Kosmas C, Skopelitis H, Gouveris P, Kopterides P, Loukeris D, Sigala F, Zorbala-Sypsa A, Felekouras E, Papalambros E. Tsavaris N, et al. Invest New Drugs. 2005 Aug;23(4):369-75. doi: 10.1007/s10637-005-1446-y. Invest New Drugs. 2005. PMID: 16012797 Clinical Trial. - Activity of raltitrexed and gemcitabine in advanced pancreatic cancer.
Kralidis E, Aebi S, Friess H, Büchler MW, Borner MM. Kralidis E, et al. Ann Oncol. 2003 Apr;14(4):574-9. doi: 10.1093/annonc/mdg150. Ann Oncol. 2003. PMID: 12649104 Clinical Trial. - Gemcitabine combined with oxaliplatin in advanced pancreatic adenocarcinoma: final results of a GERCOR multicenter phase II study.
Louvet C, André T, Lledo G, Hammel P, Bleiberg H, Bouleuc C, Gamelin E, Flesch M, Cvitkovic E, de Gramont A. Louvet C, et al. J Clin Oncol. 2002 Mar 15;20(6):1512-8. doi: 10.1200/JCO.2002.20.6.1512. J Clin Oncol. 2002. PMID: 11896099 Clinical Trial. - The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
Hind D, Tappenden P, Tumur I, Eggington S, Sutcliffe P, Ryan A. Hind D, et al. Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574 Review. - Second-line therapy for advanced pancreatic cancer: a review of the literature and future directions.
Custodio A, Puente J, Sastre J, Díaz-Rubio E. Custodio A, et al. Cancer Treat Rev. 2009 Dec;35(8):676-84. doi: 10.1016/j.ctrv.2009.08.012. Epub 2009 Sep 15. Cancer Treat Rev. 2009. PMID: 19758760 Review.
Cited by
- Pancreatic cancer: New hopes after first line treatment.
Aroldi F, Bertocchi P, Savelli G, Rosso E, Zaniboni A. Aroldi F, et al. World J Gastrointest Oncol. 2016 Sep 15;8(9):682-7. doi: 10.4251/wjgo.v8.i9.682. World J Gastrointest Oncol. 2016. PMID: 27672426 Free PMC article. Review. - Role of taxanes in pancreatic cancer.
Belli C, Cereda S, Reni M. Belli C, et al. World J Gastroenterol. 2012 Sep 7;18(33):4457-65. doi: 10.3748/wjg.v18.i33.4457. World J Gastroenterol. 2012. PMID: 22969215 Free PMC article. - Combination 5-fluorouracil, folinic acid and cisplatin (LV5FU2-CDDP) followed by gemcitabine or the reverse sequence in metastatic pancreatic cancer: final results of a randomised strategic phase III trial (FFCD 0301).
Dahan L, Bonnetain F, Ychou M, Mitry E, Gasmi M, Raoul JL, Cattan S, Phelip JM, Hammel P, Chauffert B, Michel P, Legoux JL, Rougier P, Bedenne L, Seitz JF; Fédération Francophone de Cancérologie Digestive. Dahan L, et al. Gut. 2010 Nov;59(11):1527-34. doi: 10.1136/gut.2010.216135. Gut. 2010. PMID: 20947887 Free PMC article. Clinical Trial. - High expression of erythropoietin-producing hepatoma cell line-B2 (EphB2) predicts the efficiency of the Qingyihuaji formula treatment in pancreatic cancer CFPAC-1 cells through the EphrinB1-EphB2 pathway.
Hua YQ, Chen Z, Meng ZQ, Chen H, Shen JG, Wang K, Peng W, Shen YH, Liu LM. Hua YQ, et al. Oncol Lett. 2014 Jul;8(1):17-24. doi: 10.3892/ol.2014.2134. Epub 2014 May 12. Oncol Lett. 2014. PMID: 24959213 Free PMC article. - Formulation of Folate-Modified Raltitrexed-Loaded Nanoparticles for Colorectal Cancer Theranostics.
Rosch JG, DuRoss AN, Landry MR, Sun C. Rosch JG, et al. Pharmaceutics. 2020 Feb 5;12(2):133. doi: 10.3390/pharmaceutics12020133. Pharmaceutics. 2020. PMID: 32033317 Free PMC article.
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F on behalf of the EORTC Quality of Life Study Group (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 2: 319–325 - PubMed
- Ajani JA, Welch SR, Raber MN, Fields WS, Krakoff IH (1990) Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest 8: 147–159 - PubMed
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson III AB (2002) Phase III study of gemcitabine in combination with fluorouracil vs gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20: 3270–3275 - PubMed
- Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA, for the Marimastat Pancreatic Cancer Study Group (2001) Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol 19: 3447–3455 - PubMed
- Bramhall SR, Schultz J, Nemunaitis J, Brown PD, Baillet M, Buckels JAC (2002) A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 87: 161–167 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical