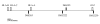A study of 82 extended HLA haplotypes in HFE-C282Y homozygous hemochromatosis subjects: relationship to the genetic control of CD8+ T-lymphocyte numbers and severity of iron overload - PubMed (original) (raw)
A study of 82 extended HLA haplotypes in HFE-C282Y homozygous hemochromatosis subjects: relationship to the genetic control of CD8+ T-lymphocyte numbers and severity of iron overload
Eugénia Cruz et al. BMC Med Genet. 2006.
Abstract
Background: It has been recently demonstrated that CD8+ T-lymphocyte numbers are genetically transmitted in association with the MHC class I region. The present study was designed with the objective of narrowing the region associated with the setting of CD8+ T-lymphocyte numbers in a population of C282Y homozygous hemochromatosis subjects, in whom a high prevalence of abnormally low CD8+ T-lymphocyte counts has been described.
Methods: The study includes 43 C282Y homozygous subjects fully characterized both phenotypically and genotypically. Clinical characterization includes measurements of iron parameters at diagnosis (transferrin saturation and serum ferritin), total body iron stores and T-cell immunophenotyping determined by flow cytometry. Genetic characterization includes HLA class I alleles (A, B and C) and four additional microsatellite markers (D6S265, D6S2222, D6S105 and D6S2239) spanning 5 Megabases in the 6p21.3 region.
Results: Eighty-two extended C282Y carrying haplotypes were defined. Single-locus analysis revealed that the HLA-A region was associated with CD8+ T-cell numbers. Multivariate analysis showed that the combinations of the most common HLA-A alleles (HLA-A*03, -A*02 and -A*01) were associated with significantly lower numbers of CD8+ T-lymphocytes (0.30 +/- 0.14 x 106/ml), in comparison with subjects carrying only one copy of those alleles (0.46 +/- 0.19 x 106/ml) and subjects without any copy of those alleles (0.79 +/- 0.15 x 106/ml;p = 0.0001). No differences were observed in CD8+ T-cell counts among control subjects carrying the same combinations of HLA-A alleles (0.47 +/- 0.14; 0.45 +/- 0.21 and 0.41 +/- 0.17 x 106/ml, respectively), therefore not supporting a direct effect of HLA specificity but rather an indirect association with a locus close to HLA-A. Multivariate analysis showed that the combination of the most common HLA-A alleles also have an impact on the clinical expression of HH in terms of iron stores, in males(p = 0.0009).
Conclusion: The present study provides evidence supporting an inextricable link between extended HLA haplotypes, CD8+ T-lymphocyte numbers and severity of iron overload in hereditary hemochromatosis(HH). It gives additional information to better define a candidate region involved in the regulation of CD8+ T-lymphocyte numbers. A new evolutionary hypothesis concerning the inheritance of the phenotype of low CD8+ T-lymphocyte numbers associated with particular ancestral HLA haplotypes carrying the C282Y mutation and its implication on the clinical heterogeneity of HH is discussed.
Figures
Figure 1
Physical map of the polymorphic markers used in the present study and their relative localization (at scale) in relation to HLA-A, B and C and HFE.
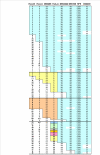
Figure 2
Multi-locus map of C282Y carrying haplotypes from a region spanning 5 Megabases from HLA-B to D6S2239. The allele specificity in each locus is represented by numbers corresponding to the size of the amplified DNA fragments. Color boxes represent conserved haplotype areas. n.d.: not determined.
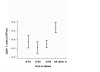
Figure 3
Mean values ± standard error of the mean of the CD8+ T-lymphocyte numbers according to the presence of different HLA-A alleles among haplotypes carrying the C282Y mutation. Haplotypes carrying the HLA-A*01 are 13, the A*02 are 16 and the A*03 are 39. The group of "All other A" specificities include: HLA-A*11, A*23, A*24, A*25, A*26, A*29, A*31, A*32, A*33 and A*68 and are 18.
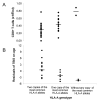
Figure 4
Distribution of the CD8+ T-lymphocyte numbers (A) and of the total body iron store (TBIS) after correction for the effect of age (residuals of TBIS on age) (B), in males, according to the HLA-A genotype of the C282Y homozygous probands, which divide subjects in the three groups: 1.subjects with two copies of the most common HLA-A alleles (A*03, A*02 and A*01; n = 27); 2.subjects with only one copy of the most common HLA-A alleles (n = 14) and 3.subjects without any copy of those alleles (n = 2).
Similar articles
- Effects of highly conserved major histocompatibility complex (MHC) extended haplotypes on iron and low CD8+ T lymphocyte phenotypes in HFE C282Y homozygous hemochromatosis patients from three geographically distant areas.
Costa M, Cruz E, Barton JC, Thorstensen K, Morais S, da Silva BM, Pinto JP, Vieira CP, Vieira J, Acton RT, Porto G. Costa M, et al. PLoS One. 2013 Nov 25;8(11):e79990. doi: 10.1371/journal.pone.0079990. eCollection 2013. PLoS One. 2013. PMID: 24282517 Free PMC article. - Low serum transferrin levels in HFE C282Y homozygous subjects are associated with low CD8(+) T lymphocyte numbers.
Macedo MF, Cruz E, Lacerda R, Porto G, de Sousa M. Macedo MF, et al. Blood Cells Mol Dis. 2005 Nov-Dec;35(3):319-25. doi: 10.1016/j.bcmd.2005.08.001. Epub 2005 Sep 1. Blood Cells Mol Dis. 2005. PMID: 16140024 - HLA haplotype A*03-B*07 in hemochromatosis probands with HFE C282Y homozygosity: frequency disparity in men and women and lack of association with severity of iron overload.
Barton JC, Wiener HW, Acton RT, Go RC. Barton JC, et al. Blood Cells Mol Dis. 2005 Jan-Feb;34(1):38-47. doi: 10.1016/j.bcmd.2004.08.022. Blood Cells Mol Dis. 2005. PMID: 15607698 - HFE, the MHC and hemochromatosis: paradigm for an extended function for MHC class I.
Cardoso CS, de Sousa M. Cardoso CS, et al. Tissue Antigens. 2003 Apr;61(4):263-75. doi: 10.1034/j.1399-0039.2003.00065.x. Tissue Antigens. 2003. PMID: 12753664 Review. - Hereditary hemochromatosis: impact of molecular and iron-based testing on the diagnosis, treatment, and prevention of a common, chronic disease.
Press RD. Press RD. Arch Pathol Lab Med. 1999 Nov;123(11):1053-9. doi: 10.5858/1999-123-1053-HH. Arch Pathol Lab Med. 1999. PMID: 10539907 Review.
Cited by
- Associations between the human MHC and sustained virologic response in the treatment of chronic hepatitis C virus infection.
Rhodes SL, Erlich H, Im KA, Wang J, Li J, Bugawan T, Jeffers L, Tong X, Su X, Rosen HR, Yee LJ, Liang TJ, Yang H; Virahep-C Study Group. Rhodes SL, et al. Genes Immun. 2008 Jun;9(4):328-33. doi: 10.1038/gene.2008.21. Epub 2008 Apr 17. Genes Immun. 2008. PMID: 18418397 Free PMC article. - Lymphocyte gene expression signatures from patients and mouse models of hereditary hemochromatosis reveal a function of HFE as a negative regulator of CD8+ T-lymphocyte activation and differentiation in vivo.
Costa M, Cruz E, Oliveira S, Benes V, Ivacevic T, Silva MJ, Vieira I, Dias F, Fonseca S, Gonçalves M, Lima M, Leitão C, Muckenthaler MU, Pinto J, Porto G. Costa M, et al. PLoS One. 2015 Apr 16;10(4):e0124246. doi: 10.1371/journal.pone.0124246. eCollection 2015. PLoS One. 2015. PMID: 25880808 Free PMC article. - Effects of highly conserved major histocompatibility complex (MHC) extended haplotypes on iron and low CD8+ T lymphocyte phenotypes in HFE C282Y homozygous hemochromatosis patients from three geographically distant areas.
Costa M, Cruz E, Barton JC, Thorstensen K, Morais S, da Silva BM, Pinto JP, Vieira CP, Vieira J, Acton RT, Porto G. Costa M, et al. PLoS One. 2013 Nov 25;8(11):e79990. doi: 10.1371/journal.pone.0079990. eCollection 2013. PLoS One. 2013. PMID: 24282517 Free PMC article. - Iron overload and immunity.
Porto G, De Sousa M. Porto G, et al. World J Gastroenterol. 2007 Sep 21;13(35):4707-15. doi: 10.3748/wjg.v13.i35.4707. World J Gastroenterol. 2007. PMID: 17729392 Free PMC article. Review. - Impact of MHC class II polymorphism on blood counts of CD4+ T lymphocytes in macaque.
Aarnink A, Garchon HJ, Puissant-Lubrano B, Blancher-Sardou M, Apoil PA, Blancher A. Aarnink A, et al. Immunogenetics. 2011 Feb;63(2):95-102. doi: 10.1007/s00251-010-0492-6. Epub 2010 Nov 18. Immunogenetics. 2011. PMID: 21086122
References
- Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potencial and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989;19:905–911. - PubMed
- van Meerwijk JP, Bianchi T, Marguerat S, MacDonald HR. Thymic lineage commitment rather than selection causes genetic variations in size of CD4 and CD8 compartments. J Immunol. 1998;160:3649–3654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials