Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor - PubMed (original) (raw)
Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor
Erinn B Rankin et al. Cancer Res. 2006.
Abstract
Inactivation of the von Hippel-Lindau tumor suppressor, pVHL, is associated with both hereditary and sporadic renal cysts and renal cell carcinoma, which are commonly thought to arise from the renal proximal tubule. pVHL regulates the protein stability of hypoxia-inducible factor (HIF)-alpha subunits and loss of pVHL function leads to HIF stabilization. The role of HIF in the development of VHL-associated renal lesions remains to be determined. To investigate the functional consequences of pVHL inactivation and the role of HIF signaling in renal epithelial cells, we used the phosphoenolpyruvate carboxykinase (PEPCK) promoter to generate transgenic mice in which Cre-recombinase is expressed in the renal proximal tubule and in hepatocytes. We found that conditional inactivation of VHL in PEPCK-Cre mutants resulted in renal cyst development that was associated with increased erythropoietin levels and polycythemia. Increased expression of the HIF target gene erythropoietin was limited to the liver, whereas expression of carbonic anhydrase 9 and multidrug resistance gene 1 was up-regulated in the renal cortex of mutant mice. Inactivation of the HIF-alpha binding partner, arylhydrocarbon receptor nuclear translocator (Arnt), but not Hif-1alpha, suppressed the development of renal cysts. Here, we present the first mouse model of VHL-associated renal disease that will provide a basis for further genetic studies to define the molecular events that are required for the progression of VHL-associated renal cysts to clear cell renal cell carcinoma.
Figures
Figure 1
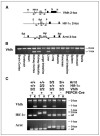
Generation of PEPCK-Cre transgenic mice. A, targeting HPRT-deficient embryonic stem cells (E14Tg2a) with a PEPCK-Cre-targeting vector by homologous recombination. Genomic maps for E14tg2a embryonic stem cells lacking the promoter and exons 1 and 2 of the HPRT gene, the PEPCK-Cre targeting vector, and the restored HPRT allele containing Cre-recombinase expression under control of the PEPCK promoter. Rectangular boxes, exons. Green box, PEPCK-Cre transgene. Black boxes, locations of probes used to identify recombined embryonic stem cell clones after digestion with BamHI (B). B, genotype analysis of embryonic stem cell clones that have undergone homologous recombination determined by Southern blot analysis. C, PEPCK-Cre expression in the kidney. 5′-Bromo-4-chloro-3-indolyl-β-
d
-galactopyranoside (Xgal) staining of a kidney tissue section from a PEPCK-Cre mouse mated to the ROSA-LacZ reporter mouse. Blue staining, cells that express PEPCK-Cre. Note that PEPCK-Cre is expressed in the renal cortex and outer medulla. D, PEPCK-Cre expression in the renal proximal tubule. Lectin and Xgal staining of kidney tissue sections from a PEPCK-Cre mouse mated to the ROSA-LacZ reporter mouse. LTA and BSAII stain the S1, S2, and S3 segments of the proximal tubule located in the renal cortex (S1, S2) and outer medulla (S3). Xgal staining indicates cells that express PEPCK-Cre. Note that ~70% of LTA-positive and 100% of BSAII-positive cells stain for Xgal. E, PEPCK-Cre is not expressed in the renal distal tubule or the medullary thick ascending limb of Henle. Localization of Xgal staining (blue) with THP expression (brown) in the renal cortex (top) and outer medulla (bottom) of PEPCK-Cre/ROSA LacZ mice. Magnification, ×200.
Figure 2
Generation of mice with Vhlh, Vhlh and _Hif-1_α, or Vhlh and Arnt deletion in the renal cortex. A, genomic maps of the Vhlh (2-lox), _Hif-1_α (2-lox), and Arnt (3-lox) conditional alleles. Rectangular boxes, exons; gray, exon targeted for deletion; black, all other exons. Gray arrows, LoxP sites. Black arrows, the location of primers used to amplify the nonrecombined (2-lox or 3-lox), recombined (1-lox), and wild-type (wt) alleles. H, HindIII; N, NcoI; E, EcoR1; P, PstI; Bgl, BglII; neo, neomycin selection cassette. B, genotype analysis of tissues collected from a PEPCK-Vhlh mutant mouse by genomic PCR. 2-Lox, nonrecombined allele; 1-lox, recombined allele. C, genotype analysis of PEPCK mutant mice by genomic PCR. T, tail; K, kidney cortex; 2, 2-lox; 3, 3-lox; +, wild type (+ for PEPCK-Cre, presence of the PEPCK-Cre transgene).
Figure 3
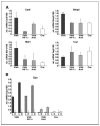
HIF target gene expression in PEPCK-Cre mutant mice. Relative expression levels for HIF target genes in the renal cortex or liver of 2-month-old male mice determined by real-time PCR. Expression levels were normalized to 18S. A, inactivation of Vhlh in PEPCK-Cre mutant mice induces HIF target gene expression in the renal cortex. Columns, average mRNA transcript level of three mice for the indicated genotype; bars, SD. *, a significant increase or decrease in target gene expression compared with control mice (Cre—) determined by Student’s t test (*, P < 0.05; **, P < 0.001). B, Epo expression in PEPCK-Vhlh and PEPCK-Vhlh/Hif-1α mutant mice is restricted to the liver. Columns, relative Epo mRNA expression levels in the liver (L) or renal cortex (K) of an individual mouse for the indicated genotype.
Figure 4
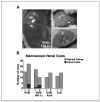
Development of macroscopic renal cysts in PEPCK-Vhlh mutant mice is ARNT dependent. A, photographs of PEPCK-Vhlh and PEPCK-Vhlh/Hif-1α mutant kidneys. Arrows, location of macroscopic renal cysts. B, incidence of macroscopic renal cysts observed in PEPCK-Cre mutant mice. Columns, number of mice with normal kidney (gray) or renal cysts (black) for a given genotype at 2 to 11 months of age (group 1) and 12 to 25 months of age (group 2). χ2 Test revealed that there was no significant difference in the number renal cysts that developed between PEPCK-Vhlh and PEPCK-Vhlh/Hif-1α mutant mice >12 months of age.
Figure 5
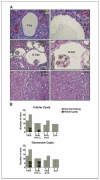
Incidence of renal microcysts in PEPCK-Cre mutant kidneys. A, H&E staining of kidney sections from PEPCK-Cre mutant and control mice. Bright-field photographs of tubular and glomerular cysts from PEPCK-Vhlh and PEPCK-Vhlh/Hif-1α mutant mice at ×200 (left) and ×600 (right) magnification and renal cortex from PEPCK-Vhlh/Arnt and control (Cre—) mice at ×200 magnification. T-Cy, tubular cyst; G-Cy, glomerular cyst. B, incidence of microscopic cysts observed in the renal cortex of PEPCK-Cre mutant mice. Columns, number of mice with normal (gray) or cystic (black) renal cortex. White line, number of PEPCK-Vhlh and PEPCK-Vhlh/Hif-1α mutant mice that developed both tubular and glomerular cysts. χ2 Test revealed there was no statistical difference in the incidence of tubular or glomerular cysts between the two mutant strains.
Figure 6
Characterization of microcysts in PEPCK-Vhlh mutant mice. A and B, genotype analysis of tubular microcysts from PEPCK-Vhlh mutant kidneys. A, bright-field photographs of a microcyst before and after laser microdissection and the microdissected epithelial cell linings of three microcysts collected onto the cap. Original magnification, ×400. B, Vhlh 1-lox PCR amplification of DNA isolated from the renal cortex (1), control cap (2), and epithelial cell linings of six microcysts (3). Note that the recombined Vhlh 1-lox allele is present in epithelial cells lining microcysts. C to E, renal microcysts in PEPCK-Vhlh mutant mice express molecular markers from multiple segments of the nephron. C, lectin staining with fluorescein-labeled LTA (green). Note that tubular cysts 1 to 3 are LTA negative and tubular cyst 4 is LTA positive. D, staining with the antibody against vimentin (VIM), a marker of cellular dedifferentiation, in epithelial cells lining a tubular microcyst. *, lumen of a tubular cyst. E, staining with the antibody against THP. Note epithelial cells lining the cyst express THP throughout the cytoplasm. F, staining with the antibody against VEGF in epithelial cells lining a tubular microcyst. *, lumen of a tubular cyst. G, staining for the proliferative marker, Ki67, in nuclei of cells lining a tubular microcyst. Arrows, epithelial cells that are Ki67 positive.
Similar articles
- Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice.
Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Rankin EB, et al. Mol Cell Biol. 2005 Apr;25(8):3163-72. doi: 10.1128/MCB.25.8.3163-3172.2005. Mol Cell Biol. 2005. PMID: 15798202 Free PMC article. - VHL-gene deletion in single renal tubular epithelial cells and renal tubular cysts: further evidence for a cyst-dependent progression pathway of clear cell renal carcinoma in von Hippel-Lindau disease.
Montani M, Heinimann K, von Teichman A, Rudolph T, Perren A, Moch H. Montani M, et al. Am J Surg Pathol. 2010 Jun;34(6):806-15. doi: 10.1097/PAS.0b013e3181ddf54d. Am J Surg Pathol. 2010. PMID: 20431476 - Identification of cyclin D1 and other novel targets for the von Hippel-Lindau tumor suppressor gene by expression array analysis and investigation of cyclin D1 genotype as a modifier in von Hippel-Lindau disease.
Zatyka M, da Silva NF, Clifford SC, Morris MR, Wiesener MS, Eckardt KU, Houlston RS, Richards FM, Latif F, Maher ER. Zatyka M, et al. Cancer Res. 2002 Jul 1;62(13):3803-11. Cancer Res. 2002. PMID: 12097293 - The von Hippel-Lindau tumor suppressor gene and kidney cancer.
Kaelin WG Jr. Kaelin WG Jr. Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6290S-5S. doi: 10.1158/1078-0432.CCR-sup-040025. Clin Cancer Res. 2004. PMID: 15448019 Review. - Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer.
Maynard MA, Ohh M. Maynard MA, et al. Am J Nephrol. 2004 Jan-Feb;24(1):1-13. doi: 10.1159/000075346. Epub 2003 Dec 3. Am J Nephrol. 2004. PMID: 14654728 Review.
Cited by
- Deletion of von Hippel-Lindau protein converts renin-producing cells into erythropoietin-producing cells.
Kurt B, Paliege A, Willam C, Schwarzensteiner I, Schucht K, Neymeyer H, Sequeira-Lopez ML, Bachmann S, Gomez RA, Eckardt KU, Kurtz A. Kurt B, et al. J Am Soc Nephrol. 2013 Feb;24(3):433-44. doi: 10.1681/ASN.2012080791. Epub 2013 Feb 7. J Am Soc Nephrol. 2013. PMID: 23393316 Free PMC article. - Retinoic Acid Signaling Coordinates Macrophage-Dependent Injury and Repair after AKI.
Chiba T, Skrypnyk NI, Skvarca LB, Penchev R, Zhang KX, Rochon ER, Fall JL, Paueksakon P, Yang H, Alford CE, Roman BL, Zhang MZ, Harris R, Hukriede NA, de Caestecker MP. Chiba T, et al. J Am Soc Nephrol. 2016 Feb;27(2):495-508. doi: 10.1681/ASN.2014111108. Epub 2015 Jun 24. J Am Soc Nephrol. 2016. PMID: 26109319 Free PMC article. - Pseudogene GSTM3P1 derived long non-coding RNA promotes ischemic acute kidney injury by target directed microRNA degradation of kidney-protective mir-668.
Wei Q, Huang J, Livingston MJ, Wang S, Dong G, Xu H, Zhou J, Dong Z. Wei Q, et al. Kidney Int. 2024 Oct;106(4):640-657. doi: 10.1016/j.kint.2024.06.027. Epub 2024 Jul 27. Kidney Int. 2024. PMID: 39074555 - Loss of vhl in the zebrafish pronephros recapitulates early stages of human clear cell renal cell carcinoma.
Noonan HR, Metelo AM, Kamei CN, Peterson RT, Drummond IA, Iliopoulos O. Noonan HR, et al. Dis Model Mech. 2016 Aug 1;9(8):873-84. doi: 10.1242/dmm.024380. Dis Model Mech. 2016. PMID: 27491085 Free PMC article. - Disruption of tubular Flcn expression as a mouse model for renal tumor induction.
Chen J, Huang D, Rubera I, Futami K, Wang P, Zickert P, Khoo SK, Dykema K, Zhao P, Petillo D, Cao B, Zhang Z, Si S, Schoen SR, Yang XJ, Zhou M, Xiao GQ, Wu G, Nordenskjöld M, Tauc M, Williams BO, Furge KA, Teh BT. Chen J, et al. Kidney Int. 2015 Nov;88(5):1057-69. doi: 10.1038/ki.2015.177. Epub 2015 Jun 17. Kidney Int. 2015. PMID: 26083655
References
- Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20. - PubMed
- Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–67. - PubMed
- Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. - PubMed
- Iliopoulos O, Kibel A, Gray S, Kaelin WG., Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases