Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies - PubMed (original) (raw)
Comparative Study
Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies
Jing-Qiong Kang et al. J Neurosci. 2006.
Abstract
With a worldwide incidence as high as 6.7% of children, febrile seizures are one of the most common reasons for seeking pediatric care, but the mechanisms underlying generation of febrile seizures are poorly understood. Febrile seizures have been suspected to have a genetic basis, and recently, mutations in GABAA receptor and sodium channel genes have been identified that are associated with febrile seizures and generalized seizures with febrile seizures plus pedigrees. Pentameric GABAA receptors mediate the majority of fast synaptic inhibition in the brain and are composed of combinations of alpha(1-6), beta(1-3), and gamma(1-3) subunits. In alphabetagamma2 GABAA receptors, the gamma2 subunit is critical for receptor trafficking, clustering, and synaptic maintenance, and mutations in the gamma2 subunit have been monogenically associated with autosomal dominant transmission of febrile seizures. Here, we report that whereas trafficking of wild-type alpha1beta2gamma2 receptors was slightly temperature dependent, trafficking of mutant alpha1beta2gamma2 receptors containing gamma2 subunit mutations [gamma2(R43Q), gamma2(K289M), and gamma2(Q351X)] associated with febrile seizures was highly temperature dependent. In contrast, trafficking of mutant alpha1beta2gamma2 receptors containing an alpha1 subunit mutation [alpha1(A322D)] not associated with febrile seizures was not highly temperature dependent. Brief increases in temperature from 37 to 40 degrees C rapidly (<10 min) impaired trafficking and/or accelerated endocytosis of heterozygous mutant alpha1beta2gamma2 receptors containing gamma2 subunit mutations associated with febrile seizures but not of wild-type alpha1beta2gamma2 receptors or heterozygous mutant alpha1(A322D)beta2gamma2 receptors, suggesting that febrile seizures may be produced by a temperature-induced dynamic reduction of susceptible mutant surface GABAA receptors in response to fever.
Figures
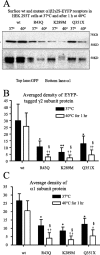
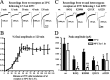
Figure 1.
Elevated temperature further reduced surface protein expression of mutant γ2S subunit-containing GABAA receptors. A, Western blot analysis of biotinylated γ2S and α1 subunit surface proteins from HEK293T cells expressing wild-type and heterozygous mutant α1β2γ2S-EYFP receptors incubated at 37 or 40°C for 1 h. The cell membranes were biotinylated, and equal amounts of protein were conjugated with beads and loaded, and the membranes were immunoblotted with mouse monoclonal anti-GFP and anti-α1 antibodies. B, Quantification of Western blots of biotinylated EYFP-tagged γ2S subunit surface protein from HEK293T cells expressing wild-type and mutant α1β2γ2S-EYFP receptors incubated at different temperatures.C, Quantification of Western blots of biotinylated α1 subunit surface protein from HEK293T cells expressing wild-type and mutant GABAA α1β2γ2S-EYFP receptors incubated at different temperatures. B, C, In all groups, data represent the mean ± SEM (n = 4; *p < 0.05, **p < 0.01 vs wild type at 37°C; ††p < 0.01 vs wild type at 40°C; §p < 0.05 vs the same mutation at 37°C; two-tailed unpaired Student’s t test).
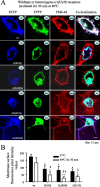
Figure 2.
Elevated temperature rapidly increased intracellular retention of mutant γ2S subunit-containing GABAA receptors. A, Confocal microscopy images of fluorescence-tagged wild-type and mutant γ2S subunit-containing α1β2γ2S receptors and α1(A322D) subunit-containing α1β2γ2S receptors in COS-7 cells after a 30 min incubation at 40°C. wt, ECFP-tagged wild-type subunits; mut, EYFP-tagged mutant subunits; mem, membrane marker FM4-64; co, colocalization of all ECFP, EYFP, and FM4-64 channels. With heterozygous expression, mutant and wild-type γ2S(R43Q), γ2S(K289M), and γ2S(Q351X) subunits and mutant α1(A322D), but not wild-type α1, subunits were localized intracellularly in compartments that had the morphology of the ER and colocalized with the ECFP-ER marker (data not shown). The loss of surface receptor illustrated here was an extreme example to illustrate the point. Other cells showed less extensive loss of surface receptor.B, Total membrane surface receptor fluorescence pixel intensity values of heterozygous α1β2γ2S(R43Q), α1β2γ2S(K289M), and α1β2γ2S(Q351X) receptors were reduced after incubation at 37°C compared with wild-type receptors (filled bars) and were further reduced after a 30 min incubation at 40°C (open bars). In all groups, data represent the mean ± SEM (n = 19–23 cells from 5 transfections; *p < 0.05 vs wild type at 37°C;†p < 0.05,††p < 0.01 vs wild type at 40°C;§p < 0.05,§§p < 0.01 vs the same mutation at 37°C; two-tailed unpaired Student’s t test).
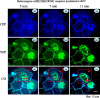
Figure 3.
Reduction of surface receptor and increased intracellular retention with elevated temperature in mutant γ2(K289M) subunit-containing α1β2γ2 receptors was rapid and dynamic in heterologous cells. The representative images illustrate the rapid time course of the reduction of heterozygous α1β2γ2S-ECFP/γ2S(K289M)-EYFP surface receptors in COS-7 cells. Cells were cotransfected with heterozygous α1β2γ2S/γ2S(K289M) receptors with the wild-type γ2S tagged with ECFP and the mutant γ2S subunit tagged with EYFP. The receptors displayed a merged aqua color both on the surface and intracellularly after incubation at 40°C for 5 min (boxed area). With incubation at 40°C over a rapid time course, the fluorescence intensity of the surface receptors progressively diminished (7 and 11 min, bottom panel, red boxes) with loss of cyan color on the cell surface (changes of cyan color in the red box, red arrow), and the intracellular fluorescence intensity progressively increased with the accumulation of intracellular receptors (changes of cyan color of the coregistered image in the red box; red double arrow). wt, ECFP-tagged wild-type subunits; mut, EYFP-tagged mutant subunits; co, colocalization of all ECFP and EYFP channels.
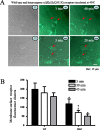
Figure 4.
Rapid reduction of heterozygous α1β2γ2/α1β2γ2(Q351X) receptors with elevated temperature on the membrane surface of hippocampal neurons.A, Heterozygous α1β2γ2L-pHluorin/α1β2γ2L(Q351X)-pHluorin receptor on the surface of rat hippocampal neurons was reduced rapidly by temperature elevation to 40°C. As illustrated in the left panels, neurons were cotransfected with heterozygous α1β2γ2L-pHluorin/α1β2γ2L(Q351X)-pHluorin receptors, and receptors were imaged as puncta on the surface of neurons. With incubation at 40°C, the fluorescent puncta were reduced, with loss or fading of fluorescence on the cell surface (red arrows). TI, Transmitted image; wt, wild-type α1β2γ2L-pHluorin receptors; mut, heterozygous mutant α1β2γ2L-pHluorin/α1β2γ2L(Q351X)-pHluorin receptors. B, Membrane surface fluorescence clusters of heterozygous mutant α1β2γ2L-pHluorin/α1β2γ2L(Q351X)-pHluorin receptors were reduced after incubation at 40°C at different times compared with wild-type receptors measured over equivalent areas and were further reduced after a 20 min incubation at 40°C compared with the receptor fluorescence puncta in the same regions. In all groups, data represent the mean ± SEM (n = 10–14 neurons from 4 transfections; *p < 0.05 vs wild type at the same time points;†p < 0.05 vs the same mutation after incubation at 40°C for 3 min; two-tailed unpaired Student’s_t_ test).
Figure 5.
Both wild-type and mutant γ2S subunit-containing GABAA receptors demonstrated a trafficking/recycling deficiency with elevated temperature.A, Representative currents are presented from a single cell expressing wild-type α1β2γ2S receptors recovering at 25°C from 2.5 h of incubation at 40°C. B, Wild-type receptor peak current amplitudes increased over a 45 min time course at 25°C and then stabilized after incubation at 40°C for 2.5 h. The ordinate denotes current amplitudes at each time point over the current amplitude obtained at 120 min after incubation at 25°C. Data were averaged from four cells.C, Representative currents are presented that were recorded at room temperature from cells that were incubated at 40°C for 1 h.D, Mutant γ2S subunit-containing receptors challenged with 40°C had reduced peak currents compared with wild-type and with the same mutant γ2S subunit-containing receptors at 37°C. In each group, data represent the mean ± SEM (n = 12–17; *p < 0.05 vs wild type at 37°C;†p < 0.05 and††p < 0.01 vs wild type at 40°C;§p < 0.05 and§§§p < 0.001 vs the same mutations at 37°C; two-tailed unpaired Student’s t test). All currents were recorded in HEK293T cells under lifted whole-cell configurations and were voltage clamped at −50 mV.
Comment in
- When is hot not so hot? Fever reduces brain inhibition.
Olsen RW. Olsen RW. Epilepsy Curr. 2006 Sep-Oct;6(5):167-9. doi: 10.1111/j.1535-7511.2006.00134.x. Epilepsy Curr. 2006. PMID: 17260048 Free PMC article. No abstract available.
Similar articles
- Three epilepsy-associated GABRG2 missense mutations at the γ+/β- interface disrupt GABAA receptor assembly and trafficking by similar mechanisms but to different extents.
Huang X, Hernandez CC, Hu N, Macdonald RL. Huang X, et al. Neurobiol Dis. 2014 Aug;68:167-79. doi: 10.1016/j.nbd.2014.04.015. Epub 2014 May 4. Neurobiol Dis. 2014. PMID: 24798517 Free PMC article. - The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression.
Kang JQ, Shen W, Macdonald RL. Kang JQ, et al. J Neurosci. 2009 Mar 4;29(9):2845-56. doi: 10.1523/JNEUROSCI.4772-08.2009. J Neurosci. 2009. PMID: 19261880 Free PMC article. - SAHA (Vorinostat) Corrects Inhibitory Synaptic Deficits Caused by Missense Epilepsy Mutations to the GABAA Receptor γ2 Subunit.
Durisic N, Keramidas A, Dixon CL, Lynch JW. Durisic N, et al. Front Mol Neurosci. 2018 Mar 23;11:89. doi: 10.3389/fnmol.2018.00089. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29628874 Free PMC article. - Molecular Pathogenic Basis for GABRG2 Mutations Associated With a Spectrum of Epilepsy Syndromes, From Generalized Absence Epilepsy to Dravet Syndrome.
Kang JQ, Macdonald RL. Kang JQ, et al. JAMA Neurol. 2016 Aug 1;73(8):1009-16. doi: 10.1001/jamaneurol.2016.0449. JAMA Neurol. 2016. PMID: 27367160 Free PMC article. Review. - Mutant GABA(A) receptor subunits in genetic (idiopathic) epilepsy.
Hirose S. Hirose S. Prog Brain Res. 2014;213:55-85. doi: 10.1016/B978-0-444-63326-2.00003-X. Prog Brain Res. 2014. PMID: 25194483 Review.
Cited by
- When is hot not so hot? Fever reduces brain inhibition.
Olsen RW. Olsen RW. Epilepsy Curr. 2006 Sep-Oct;6(5):167-9. doi: 10.1111/j.1535-7511.2006.00134.x. Epilepsy Curr. 2006. PMID: 17260048 Free PMC article. No abstract available. - Mutational consequences of aberrant ion channels in neurological disorders.
Kumar D, Ambasta RK, Kumar P. Kumar D, et al. J Membr Biol. 2014 Nov;247(11):1083-127. doi: 10.1007/s00232-014-9716-2. Epub 2014 Aug 14. J Membr Biol. 2014. PMID: 25119057 Review. - Heat induced temperature dysregulation and seizures in Dravet Syndrome/GEFS+ Gabrg2+/Q390X mice.
Warner TA, Liu Z, Macdonald RL, Kang JQ. Warner TA, et al. Epilepsy Res. 2017 Aug;134:1-8. doi: 10.1016/j.eplepsyres.2017.04.020. Epub 2017 Apr 30. Epilepsy Res. 2017. PMID: 28505490 Free PMC article. - Febrile seizures: recent developments and unanswered questions.
Pavlidou E, Hagel C, Panteliadis C. Pavlidou E, et al. Childs Nerv Syst. 2013 Nov;29(11):2011-7. doi: 10.1007/s00381-013-2224-3. Epub 2013 Jul 12. Childs Nerv Syst. 2013. PMID: 23846392 Review. - Identification of two novel and one rare mutation in DYRK1A and prenatal diagnoses in three Chinese families with intellectual Disability-7.
Huang C, Luo H, Zeng B, Feng C, Chen J, Yuan H, Huang S, Yang B, Zou Y, Liu Y. Huang C, et al. Front Genet. 2023 Dec 20;14:1290949. doi: 10.3389/fgene.2023.1290949. eCollection 2023. Front Genet. 2023. PMID: 38179410 Free PMC article.
References
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud’homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E (2001). First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet 28:46–48. - PubMed
- Baulac S, Gourfinkel-An I, Nabbout R, Huberfeld G, Serratosa J, LeGuern E, Baulac M (2004). Fever, genes, and epilepsy. Lancet Neurol 3:421–430. - PubMed
- Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ (2003). Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci 23:6826–6836. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical