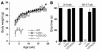Prelamin A and lamin A appear to be dispensable in the nuclear lamina - PubMed (original) (raw)
. 2006 Mar;116(3):743-52.
doi: 10.1172/JCI27125.
Jennifer K Ng, Jan Lammerding, Timothy A Vickers, Margarita Meta, Nathan Coté, Bryant Gavino, Xin Qiao, Sandy Y Chang, Stephanie R Young, Shao H Yang, Colin L Stewart, Richard T Lee, C Frank Bennett, Martin O Bergo, Stephen G Young
Affiliations
- PMID: 16511604
- PMCID: PMC1386109
- DOI: 10.1172/JCI27125
Prelamin A and lamin A appear to be dispensable in the nuclear lamina
Loren G Fong et al. J Clin Invest. 2006 Mar.
Abstract
Lamin A and lamin C, both products of Lmna, are key components of the nuclear lamina. In the mouse, a deficiency in both lamin A and lamin C leads to slow growth, muscle weakness, and death by 6 weeks of age. Fibroblasts deficient in lamins A and C contain misshapen and structurally weakened nuclei, and emerin is mislocalized away from the nuclear envelope. The physiologic rationale for the existence of the 2 different Lmna products lamin A and lamin C is unclear, although several reports have suggested that lamin A may have particularly important functions, for example in the targeting of emerin and lamin C to the nuclear envelope. Here we report the development of lamin C-only mice (Lmna(LCO/LCO)), which produce lamin C but no lamin A or prelamin A (the precursor to lamin A). Lmna(LCO/LCO) mice were entirely healthy, and Lmna(LCO/LCO) cells displayed normal emerin targeting and exhibited only very minimal alterations in nuclear shape and nuclear deformability. Thus, at least in the mouse, prelamin A and lamin A appear to be dispensable. Nevertheless, an accumulation of farnesyl-prelamin A (as occurs with a deficiency in the prelamin A processing enzyme Zmpste24) caused dramatically misshapen nuclei and progeria-like disease phenotypes. The apparent dispensability of prelamin A suggested that lamin A-related progeroid syndromes might be treated with impunity by reducing prelamin A synthesis. Remarkably, the presence of a single Lmna(LCO) allele eliminated the nuclear shape abnormalities and progeria-like disease phenotypes in Zmpste24-/- mice. Moreover, treating Zmpste24-/- cells with a prelamin A-specific antisense oligonucleotide reduced prelamin A levels and significantly reduced the frequency of misshapen nuclei. These studies suggest a new therapeutic strategy for treating progeria and other lamin A diseases.
Figures
Figure 1
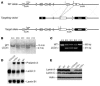
Production of a mutant Lmna allele, LmnaLCO. (A) A sequence-replacement vector was used to remove intron 11 and the last 150 nucleotides of exon 11. Unexpectedly, this mutation eliminated the splicing event required to produce prelamin A. Exons are shown as boxes. Locations of the PCR primers (i and ii) and the 5′ flanking probe for Southern blots are shown. tk, thymidine kinase gene (for negative selection). (B) Southern blot detection of the LmnaLCO (LCO/+) allele in _Eco_RI-cleaved genomic DNA from ES cells. (C) PCR identification of wild-type and LmnaLCO alleles. Shown are results with wild-type (A10, C12) and LmnaLCO/+ (B10, B11, B12) ES cell clones from gene-targeting experiments. (D) Northern blot of total RNA from Lmna+/+ (+/+), LmnaLCO/+ (LCO/+), and Lmna+/+ (LCO/LCO) fibroblasts; the blot was hybridized with a mouse Lmna cDNA probe that detects both prelamin A and lamin C transcripts and a mouse Lmnb1 cDNA probe that detects lamin B1, a closely related lamin protein. (E) Western blots of 3 different wild-type, 2 heterozygous, and 1 homozygous primary fibroblast cell lines at the same passage number with a polyclonal antibody against lamin A/C and actin. A minor form of lamin A lacking exon 10 has been reported previously in some human cells (43). It is conceivable that this minor lamin could be synthesized, but this would require splicing from exon 9 to the exon 11–12 fusion. We have not identified this minor splice variant in the lamin C–only fibroblasts.
Figure 2
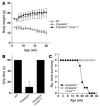
Weight gain and grip strength is normal in Lmna+/+ and LmnaLCO/ – mice. (A) Female LmnaLCO/ – (n = 9) and Lmna+/+ mice (n = 13) exhibit normal growth, indistinguishable from wild-type mice (n = 9). Similar results were obtained with male mice (not shown). The growth rate for female Lmna –/ – mice (n = 7) is shown for comparison. (B) Grip strength in 3- to 4-week-old and 16- to 17-week-old mice as judged by the length of time that they were able to hang upside-down from a grid (14). For 3- to 4-week-old mice, n = 7 (wild-type), 13 (Lmna –/ –), 9 (LmnaLCO/ –), and 11 (Lmna+/+). For 16-week-old mice, n = 3 (wild-type), 6 (LmnaLCO/ –), and 7 (Lmna+/+). Grip strength in Lmna –/ – mice at 16 weeks of age was not determined (ND) because the mice did not survive to this age. NS, wild type versus Lmna+/+.
Figure 3
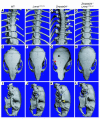
Bone abnormalities are absent in Lmna+/+ and Zmpste24 –/ –Lmna+/+ mice. Surface renderings of μCT scans of the spine and skull are shown for 28-week-old wild-type (A, E, and I), Lmna+/+ (B, F, and J), Zmpste24 –/ – (C, G, and K) and Zmpste24 –/ –Lmna+/+ mice (D, H, and L). (A–D) Thoracic spine. In the Zmpste24 –/ – mouse (C), callus around a rib fracture is indicated by a white arrow. Rib fractures were absent in Lmna+/+ and Zmpste24 –/ –Lmna+/+ mice. (E–H) Top view of skulls. Zmpste24 –/ – mice showed a loss of the zigzag appearance of the cranial sutures (black arrow) and an osteolytic lesion of the zygomatic arch (red arrow). Wild-type, Lmna+/+, and Zmpste24 –/ –Lmna+/+ skulls were normal. (I–L) Lateral view of skulls. Zmpste24 –/ – mice exhibited a small mandible and an osteolytic lesion in the posterior portion of the zygomatic arch (yellow arrow). These abnormalities were absent in wild-type, Lmna+/+, and Zmpste24 –/ –Lmna+/+ skulls.
Figure 4
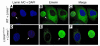
Emerin localization is normal in Lmna+/+ primary embryonic fibroblasts. The localization of lamins A and C (red) and emerin (green) in wild-type, Lmna –/ –, and Lmna+/+ fibroblasts was determined by confocal fluorescence microscopy. DAPI was used to visualize DNA. To obtain side-by-side comparisons with Lmna –/ – cells, wild-type and Lmna+/+ fibroblasts were mixed together with Lmna –/ – cells in equal proportions and plated on the same coverslip. (A–C) Wild-type cells plus Lmna –/ – cells. Cells were stained with an anti–lamin A/C antibody, and wild-type cells (arrows) were identified by red fluorescence. As expected based on the studies of Sullivan and coworkers (11), emerin was normally located within the nuclei of wild-type cells, but was mislocalized to the ER in Lmna –/ – cells. (D–F) Lmna+/+ cells plus Lmna –/ – cells. Cells were plated and stained as described for wild-type cells. Lmna+/+ cells (arrows) were identified by red fluorescence. Emerin was located in the nucleus of Lmna+/+ cells with a pattern indistinguishable from that of wild-type cells.
Figure 5
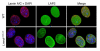
Lamin C is normally targeted to the nuclear envelope in Lmna+/+ primary embryonic fibroblasts. Wild-type and Lmna+/+ cells were incubated with antibodies against lamin A/C (red) and LAP2 (green) or DAPI to visualize DNA (blue), and the cells were examined by epifluorescence microscopy. The red fluorescence in wild-type cells identifies both lamin A and lamin C, whereas the red fluorescence in Lmna+/+ cells represents only lamin C staining. Both lamin C and LAP2 in Lmna+/+ cells were located in the nucleus and nuclear rim, and their distribution was indistinguishable from that of wild-type cells.
Figure 6
Analysis of nuclear shape in wild-type, Lmna –/ –, LmnaLCO/ –, LmnaLCO/+, and Lmna+/+ fibroblasts. The nuclear envelope of cells was visualized with antibodies against lamin B or LAP2, and the number of cells with misshapen nuclei was scored by 2 trained observers blinded to genotype. Bars show the mean frequency of misshapen nuclei; the number of misshapen nuclei and the total number of nuclei examined are shown within each bar. The graph summarizes data for 3 independent experiments (filled circles). Both LmnaLCO/+ and Lmna+/+ cells had a slight but statistically significant increase in number of misshapen nuclei compared with wild-type cells (P < 0.007, χ2 test).
Figure 7
_Lmna+/+_and _LmnaLCO/+_cells exhibit greater nuclear deformation by biaxial strain than do wild-type cells. Biaxial strain was applied to wild-type, LmnaLCO/+, Lmna+/+, and Lmna –/ – cells, and the extent of nuclear deformation was measured and expressed as a ratio of nuclear strain to membrane strain (Normalized nuclear strain). LmnaLCO/+ and Lmna+/+ cells exhibited a small but statistically significant increase in normalized nuclear strain compared with wild-type cells (P < 0.001, unpaired t test with Welch’s correction).
Figure 8
A single LmnaLCO allele reduces the number of misshapen nuclei in primary Zmpste24 –/ – mouse embryonic fibroblasts. Primary embryonic fibroblasts were isolated from embryos generated from Zmpste24+/ –LmnaLCO/+ intercrosses. The cells were stained with lamin A/C and LAP2 antibodies, and the number of cells with abnormal shaped nuclei was determined by fluorescence microscopy. Bars show the mean frequency of misshapen nuclei; the number of misshapen nuclei and the total number of nuclei examined are recorded within each bar. The bar graph summarizes data from 2 independent experiments (filled circles). P < 0.001, Zmpste24 –/ – versus Zmpste24 –/ –LmnaLCO/+ or Zmpste24 –/ –Lmna+/+.
Figure 9
Growth rates, grip strength, and survival of Zmpste24 –/ –LmnaLCO/+ and Zmpste24 –/ – mice. Compound heterozygotes (Zmpste24+/ –LmnaLCO/+) were intercrossed to generate littermate Zmpste24 –/ –LmnaLCO/+ and Zmpste24 –/ –Lmna+/+ mice. (A) The growth rate of male Zmpste24 –/ – mice (n = 4) was retarded, whereas Zmpste24 –/ –LmnaLCO/+ mice (n = 5) exhibited normal growth indistinguishable from that of wild-type mice (n = 7). Similar results were obtained with male Zmpste24 –/ –Lmna+/+ mice (not shown). (B) Grip strength in 17-week-old wild-type (n = 7), Zmpste24 –/ – (n = 4), and Zmpste24 –/ –LmnaLCO/+ mice (n = 5), as judged by the length of time that they were able to hang upside-down from a grid (14). P < 0.001, t test, Zmpste24 –/ – versus Zmpste24 –/ –LmnaLCO/+. (C) The survival rates were followed over a 30-week period. By 26 weeks of age, all of the Zmpste24 –/ – mice had died (or were euthanized at the request of the veterinary staff). In contrast, all of the Zmpste24 –/ – mice with a single LmnaLCO allele were alive and exhibiting normal health. n = 6 mice per group.
Figure 10
Western blots of extracts from immortalized wild-type and Zmpste24 –/ – embryonic fibroblasts after treatment with ISIS 359445, a prelamin A–specific antisense oligonucleotide. Cells were transfected with the antisense oligonucleotide, and after 48 hours lamin A (in wild-type cells) or prelamin A (in Zmpste24 –/ – cells) levels were measured by Western blot. (A) Wild-type cells. ISIS 359445 inhibited lamin A protein levels in a concentration-dependent manner, whereas a nonspecific control oligonucleotide had no effect. (B) Zmpste24 –/ – cells. ISIS 359445 inhibited prelamin A protein levels as measured using a prelamin A antibody, whereas the control oligonucleotide had no effect.
Figure 11
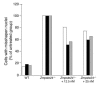
Improved nuclear shape in immortalized Zmpste24 –/ – fibroblasts treated with an antisense oligonucleotide specific for mouse prelamin A (ISIS 359445). Zmpste24 –/ – cells were treated with ISIS 359445; after 72 hours, the cells were stained with antibodies against lamin A/C and LAP2, and the number of cells with misshapen nuclei was scored by 3 trained observers blinded to genotype and treatment. Bars show the frequency of misshapen nuclei as a percentage of untreated Zmpste24 –/ – cells for each individual observer (observer 1, white bars; observer 2, black bars; observer 3, gray bars). There was a statistically significant decrease in the number of misshapen nuclei for cells treated with 12.5 and 25 nM oligonucleotide compared with untreated Zmpste24 –/ – cells for each observer (P < 0.001, χ2 test). The average number of cells with misshapen nuclei in the untreated Zmpste24 –/ – cells was 78.5 cells. The higher baseline level of misshapen nuclei in the Zmpste24 –/ – fibroblasts in this experiment compared with Figure 8 is likely due to the fact that the cells were immortalized. We have previously noted much higher levels of misshapen nuclei in immortalized Zmpste24 –/ – fibroblasts than in primary Zmpste24 –/ – fibroblasts (44).
Comment in
- Good news in the nuclear envelope: loss of lamin A might be a gain.
Scaffidi P, Misteli T. Scaffidi P, et al. J Clin Invest. 2006 Mar;116(3):632-4. doi: 10.1172/JCI27820. J Clin Invest. 2006. PMID: 16511598 Free PMC article. Review.
Similar articles
- Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice.
Fong LG, Ng JK, Meta M, Coté N, Yang SH, Stewart CL, Sullivan T, Burghardt A, Majumdar S, Reue K, Bergo MO, Young SG. Fong LG, et al. Proc Natl Acad Sci U S A. 2004 Dec 28;101(52):18111-6. doi: 10.1073/pnas.0408558102. Epub 2004 Dec 17. Proc Natl Acad Sci U S A. 2004. PMID: 15608054 Free PMC article. - Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes.
Toth JI, Yang SH, Qiao X, Beigneux AP, Gelb MH, Moulson CL, Miner JH, Young SG, Fong LG. Toth JI, et al. Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12873-8. doi: 10.1073/pnas.0505767102. Epub 2005 Aug 29. Proc Natl Acad Sci U S A. 2005. PMID: 16129834 Free PMC article. - Eliminating the synthesis of mature lamin A reduces disease phenotypes in mice carrying a Hutchinson-Gilford progeria syndrome allele.
Yang SH, Qiao X, Farber E, Chang SY, Fong LG, Young SG. Yang SH, et al. J Biol Chem. 2008 Mar 14;283(11):7094-9. doi: 10.1074/jbc.M708138200. Epub 2008 Jan 4. J Biol Chem. 2008. PMID: 18178963 - Prelamin A farnesylation and progeroid syndromes.
Young SG, Meta M, Yang SH, Fong LG. Young SG, et al. J Biol Chem. 2006 Dec 29;281(52):39741-5. doi: 10.1074/jbc.R600033200. Epub 2006 Nov 7. J Biol Chem. 2006. PMID: 17090536 Review. - Prelamin A, Zmpste24, misshapen cell nuclei, and progeria--new evidence suggesting that protein farnesylation could be important for disease pathogenesis.
Young SG, Fong LG, Michaelis S. Young SG, et al. J Lipid Res. 2005 Dec;46(12):2531-58. doi: 10.1194/jlr.R500011-JLR200. Epub 2005 Oct 5. J Lipid Res. 2005. PMID: 16207929 Review.
Cited by
- Sorting nexin 6 enhances lamin a synthesis and incorporation into the nuclear envelope.
González-Granado JM, Navarro-Puche A, Molina-Sanchez P, Blanco-Berrocal M, Viana R, Font de Mora J, Andrés V. González-Granado JM, et al. PLoS One. 2014 Dec 23;9(12):e115571. doi: 10.1371/journal.pone.0115571. eCollection 2014. PLoS One. 2014. PMID: 25535984 Free PMC article. - Ancient Eukaryotic Origin and Evolutionary Plasticity of Nuclear Lamina.
Koreny L, Field MC. Koreny L, et al. Genome Biol Evol. 2016 Sep 19;8(9):2663-71. doi: 10.1093/gbe/evw087. Genome Biol Evol. 2016. PMID: 27189989 Free PMC article. - Restrictive dermopathy--a lethal congenital laminopathy. Case report and review of the literature.
Morais P, Magina S, Ribeiro Mdo C, Rodrigues M, Lopes JM, Thanh Hle T, Wehnert M, Guimarães H. Morais P, et al. Eur J Pediatr. 2009 Aug;168(8):1007-12. doi: 10.1007/s00431-008-0868-x. Epub 2008 Nov 20. Eur J Pediatr. 2009. PMID: 19020898 Review. - Lonafarnib and everolimus reduce pathology in iPSC-derived tissue engineered blood vessel model of Hutchinson-Gilford Progeria Syndrome.
Abutaleb NO, Atchison L, Choi L, Bedapudi A, Shores K, Gete Y, Cao K, Truskey GA. Abutaleb NO, et al. Sci Rep. 2023 Mar 28;13(1):5032. doi: 10.1038/s41598-023-32035-3. Sci Rep. 2023. PMID: 36977745 Free PMC article. - Development of a CRISPR/Cas9-based therapy for Hutchinson-Gilford progeria syndrome.
Santiago-Fernández O, Osorio FG, Quesada V, Rodríguez F, Basso S, Maeso D, Rolas L, Barkaway A, Nourshargh S, Folgueras AR, Freije JMP, López-Otín C. Santiago-Fernández O, et al. Nat Med. 2019 Mar;25(3):423-426. doi: 10.1038/s41591-018-0338-6. Epub 2019 Feb 18. Nat Med. 2019. PMID: 30778239 Free PMC article.
References
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 1993;268:16321–16326. - PubMed
- Muchir A, Worman HJ. The nuclear envelope and human disease. Physiology (Bethesda). 2004;19:309–314. - PubMed
- Hutchison CJ, Worman HJ. A-type lamins: guardians of the soma? Nat. Cell Biol. 2004;6:1062–1067. - PubMed
- Mounkes LC, Burke B, Stewart CL. The A-type lamins. Nuclear structural proteins as a focus for muscular dystrophy and cardiovascular diseases. Trends Cardiovasc. Med. 2001;11:280–285. - PubMed
- Wilson KL. The nuclear envelope, muscular dystrophy and gene expression. Trends Cell Biol. 2000;10:125–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA099506/CA/NCI NIH HHS/United States
- R01 NS059348/NS/NINDS NIH HHS/United States
- R01 AR050200/AR/NIAMS NIH HHS/United States
- AI054384/AI/NIAID NIH HHS/United States
- R01 AI054384/AI/NIAID NIH HHS/United States
- CA099506/CA/NCI NIH HHS/United States
- AR050200/AR/NIAMS NIH HHS/United States
- R01 HL082792/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous