Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons - PubMed (original) (raw)
Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons
Anton Ivanov et al. J Physiol. 2006.
Abstract
The extracellular signal-regulated kinases (ERK) signalling cascade is a key pathway that mediates the NMDA receptor (NMDAR)-dependent neuronal plasticity and survival. However, it is not clear yet how NMDARs regulate ERK activity. Stimulation of the NMDARs induces a complex modification of ERK that includes both ERK activation and inactivation and depends on particular experimental conditions. Here we show that there exists a differential restriction in the regulation of ERK activity that depends on the pool of NMDAR that was activated. The synaptic pool of NMDARs activates ERK whereas the extrasynaptic pool does not; on the contrary, it triggers a signalling pathway that results in the inactivation of ERK. As a result, simultaneous activation of both extrasynaptic and synaptic NMDAR using bath application of NMDA or glutamate (a typical protocol explored in the majority of studies) produced ERK activation that depended on the concentration of agonists and was always significantly weaker than those mediated by synaptic NMDARs. Since the activation of the extrasynaptic NMDA is attributed mainly to global release of glutamate occurring at pathological conditions including hypoxic/ischaemic insults, traumas and epileptic brain damage, the reported differential regulation of ERK cascade by NMDARs provides a unique mechanism for an early identification of the physiological and/or pathophysiological consequences of NMDAR activation. The negative regulation of the ERK activity might be one of the first signalling events determining brain injury and constitutes a putative target of new pharmacological applications.
Figures
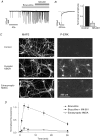
Figure 1. Activation of ERK by different subpopulations of NMDARs
A, example of voltage clamp whole cell recording of spontaneous excitatory postsynaptic currents (EPSCs) that reflect activity of the neuronal network during bath application of bicuculline and co-application of MK-801 1 min later. _V_h=−60 mV. B, neuron treatment with MK-801 in presence of bicuculline effectively inhibits synaptic NMDAR component of EPSCs. Superimposed traces illustrate spontaneous EPSCs recorded in the presence of bicuculline before (black) and after (grey) neuron incubation with MK-801. Plot shows MK-801-induced decrease of the amplitude of slow (NMDAR) component of EPSCs measured 100 ms after peak value. Mean data from 4 experiments, 5–7 events in each experiment. C, images of phosphorylated ERK1, 2 (P-ERK) and MAP2, in neurons activated via synaptic and extrasynaptic NMDARs. D, time course of ERK phosphorylation after activation of different populations of NMDARs. Abscissa indicates the time of treatment of cultures using mentioned protocols. Mean ±
s.e.m.
from 4 experiments. Bicuculline induced significantly different phosphorylation of ERK (P < 0.05) at all studied time points as compared with other experimental conditions.
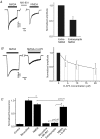
Figure 2. Differential sensitivity of P-ERK to reduction of NMDA current through extrasynaptic and entire pool of NMDARs
A, currents induced by bath activation of 10 μ
m
NMDA before and after block of the synaptic NMDARs with MK-801 (entire NMDA and extrasynaptic NMDA, respectively). B, dose dependence of the inhibition of NMDA (10 μ
m
)-induced currents by
d
-AP5. n = 7. Example shows current recorded during application of 10 μ
m
NMDA and NMDA with 10 μ
m
D-AP5. C, inhibition of the NMDAR with 20 μ
md
-AP5 does not affect the NMDA-induced ERK phosphorylation. Neurons were incubated with indicated agonists and blockers for 5 min. Mean ±
s.e.m.
from 5 experiments.
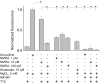
Figure 3. ERK phosphorylation at different strengths of NMDAR activation
Phosphorylation of the ERK after NMDAR activation during 5 min with bicuculline, glutamate, different concentrations of NMDA and under different experimental conditions as indicated by (+) and (−). The basal external solution was as described in Methods. n = 4.
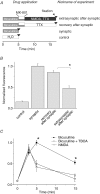
Figure 4. Activation of the extrasynaptic NMDAR induces dephosphorylation of P-ERK
A, scheme of the experiment. Horizontal bars indicate the duration of incubation of neurons with indicated drugs. B, mean data from 3 experiments showing that activation of the extrasynaptic pool of NMDAR induces dephosphorylation of P-ERK. C, ERK phosphorylation in neuronal cultures incubated during indicated times with bicuculline, bicuculline + DL-TBOA (50 μ
m
), and NMDA (10 μ
m
). Mean data from 4 experiments. Asterisks indicate values of ERK phosphorylation significantly different from others measured at the same time points (P < 0.01).
Comment in
- 2B synaptic or extrasynaptic determines signalling from the NMDA receptor.
Hardingham GE. Hardingham GE. J Physiol. 2006 May 1;572(Pt 3):614-5. doi: 10.1113/jphysiol.2006.109603. J Physiol. 2006. PMID: 16581856 Free PMC article. No abstract available.
Similar articles
- Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors.
Léveillé F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Léveillé F, et al. FASEB J. 2008 Dec;22(12):4258-71. doi: 10.1096/fj.08-107268. Epub 2008 Aug 18. FASEB J. 2008. PMID: 18711223 - 2B synaptic or extrasynaptic determines signalling from the NMDA receptor.
Hardingham GE. Hardingham GE. J Physiol. 2006 May 1;572(Pt 3):614-5. doi: 10.1113/jphysiol.2006.109603. J Physiol. 2006. PMID: 16581856 Free PMC article. No abstract available. - Mechanisms underlying dedepression of synaptic NMDA receptors in the hippocampus.
Morishita W, Malenka RC. Morishita W, et al. J Neurophysiol. 2008 Jan;99(1):254-63. doi: 10.1152/jn.01011.2007. Epub 2007 Nov 7. J Neurophysiol. 2008. PMID: 17989241 - Surface trafficking of N-methyl-D-aspartate receptors: physiological and pathological perspectives.
Groc L, Bard L, Choquet D. Groc L, et al. Neuroscience. 2009 Jan 12;158(1):4-18. doi: 10.1016/j.neuroscience.2008.05.029. Epub 2008 Jun 26. Neuroscience. 2009. PMID: 18583064 Review. - Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects.
Vizi ES, Kisfali M, Lőrincz T. Vizi ES, et al. Brain Res Bull. 2013 Apr;93:32-8. doi: 10.1016/j.brainresbull.2012.10.005. Epub 2012 Oct 23. Brain Res Bull. 2013. PMID: 23089362 Review.
Cited by
- NMDA receptor subunit composition determines beta-amyloid-induced neurodegeneration and synaptic loss.
Tackenberg C, Grinschgl S, Trutzel A, Santuccione AC, Frey MC, Konietzko U, Grimm J, Brandt R, Nitsch RM. Tackenberg C, et al. Cell Death Dis. 2013 Apr 25;4(4):e608. doi: 10.1038/cddis.2013.129. Cell Death Dis. 2013. PMID: 23618906 Free PMC article. - Calpain-mediated degradation of myocyte enhancer factor 2D contributes to excitotoxicity by activation of extrasynaptic N-methyl-D-aspartate receptors.
Wei G, Yin Y, Li W, Bito H, She H, Mao Z. Wei G, et al. J Biol Chem. 2012 Feb 17;287(8):5797-805. doi: 10.1074/jbc.M111.260109. Epub 2012 Jan 3. J Biol Chem. 2012. PMID: 22215669 Free PMC article. - Pathophysiological Ionotropic Glutamate Signalling in Neuroinflammatory Disease as a Therapeutic Target.
Fairless R, Bading H, Diem R. Fairless R, et al. Front Neurosci. 2021 Oct 21;15:741280. doi: 10.3389/fnins.2021.741280. eCollection 2021. Front Neurosci. 2021. PMID: 34744612 Free PMC article. Review. - Early phase of plasticity-related gene regulation and SRF dependent transcription in the hippocampus.
Iacono G, Altafini C, Torre V. Iacono G, et al. PLoS One. 2013 Jul 23;8(7):e68078. doi: 10.1371/journal.pone.0068078. Print 2013. PLoS One. 2013. PMID: 23935853 Free PMC article. - Homocysteine-NMDA receptor-mediated activation of extracellular signal-regulated kinase leads to neuronal cell death.
Poddar R, Paul S. Poddar R, et al. J Neurochem. 2009 Aug;110(3):1095-106. doi: 10.1111/j.1471-4159.2009.06207.x. Epub 2009 Jun 5. J Neurochem. 2009. PMID: 19508427 Free PMC article.
References
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. - PubMed
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. - PubMed
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. - PubMed
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous