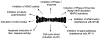Chemoprotection by sulforaphane: keep one eye beyond Keap1 - PubMed (original) (raw)
Review
Chemoprotection by sulforaphane: keep one eye beyond Keap1
Melinda C Myzak et al. Cancer Lett. 2006.
Abstract
Sulforaphane (SFN) is an isothiocyanate found in cruciferous vegetables, with particularly high levels detected in broccoli and broccoli sprouts. Over a decade ago, this phytochemical was identified as a likely chemopreventive agent based on its ability to induce Phase 2 detoxification enzymes, as well as to inhibit Phase 1 enzymes involved in carcinogen activation. Considerable attention has focused on SFN as a 'blocking' agent, with the ability to modulate the Nrf2/Keap1 pathway, but recent evidence suggests that SFN acts by numerous other mechanisms. SFN induces cell cycle arrest and apoptosis in cancer cells, inhibits tubulin polymerization, activates checkpoint 2 kinase, and inhibits histone deacetylase activity. The latter findings suggest that SFN may be effective during the post-initiation stages of carcinogenesis, as a 'suppressing' agent. Moreover, pharmacological administration of SFN may be a promising therapeutic approach to the treatment of cancers, including those characterized by increased inflammation and involving viral or bacterial-related pathologies. The present review discusses the more widely established chemoprotective mechanisms of SFN, but makes the case for additional work on mechanisms that might be of importance during later stages of carcinogenesis, beyond Keap1.
Figures
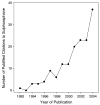
Fig. 1
Increasing interest in SFN, based on annual citations in PubMed.
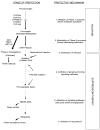
Fig. 2
SFN acts at various steps during multi-stage carcinogenesis. This figure illustrates the various ‘blocking’ and ‘suppressing’ mechanisms by which SFN inhibits multi-stage carcinogenesis. Each of the protective mechanisms (1–6) is described in further detail in the text. Adapted from a previous review [5].
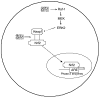
Fig. 3
SFN induces ARE expression through disruption of the Keap1–Nrf2 complex. SFN can interact directly with sulfhydryl residues on Keap1, causing Nrf2 to be released. Alternatively, SFN can activate the MAPK pathway, causing phosphorylation of Keap1 and release of Nrf2. Once released, Nrf2 enters the nucleus, where it transactivates ARE-responsive genes.
Fig. 4
Multiple mechanisms of chemoprotection by SFN. This figure combines empirically tested as well as postulated activities of SFN, based largely on in vitro studies, plus in vivo findings where available. Each of the protective mechanisms is described in further detail in the text.
Similar articles
- Multi-targeted prevention of cancer by sulforaphane.
Clarke JD, Dashwood RH, Ho E. Clarke JD, et al. Cancer Lett. 2008 Oct 8;269(2):291-304. doi: 10.1016/j.canlet.2008.04.018. Epub 2008 May 27. Cancer Lett. 2008. PMID: 18504070 Free PMC article. Review. - Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells.
Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Clarke JD, et al. Mol Nutr Food Res. 2011 Jul;55(7):999-1009. doi: 10.1002/mnfr.201000547. Epub 2011 Mar 4. Mol Nutr Food Res. 2011. PMID: 21374800 Free PMC article. - A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase.
Myzak MC, Karplus PA, Chung FL, Dashwood RH. Myzak MC, et al. Cancer Res. 2004 Aug 15;64(16):5767-74. doi: 10.1158/0008-5472.CAN-04-1326. Cancer Res. 2004. PMID: 15313918 - [Sulforaphane--a possible agent in prevention and therapy of cancer].
Tomczyk J, Olejnik A. Tomczyk J, et al. Postepy Hig Med Dosw (Online). 2010 Nov 29;64:590-603. Postepy Hig Med Dosw (Online). 2010. PMID: 21160094 Review. Polish. - Involvement of c-Jun N-terminal kinase in G2/M arrest and caspase-mediated apoptosis induced by sulforaphane in DU145 prostate cancer cells.
Cho SD, Li G, Hu H, Jiang C, Kang KS, Lee YS, Kim SH, Lu J. Cho SD, et al. Nutr Cancer. 2005;52(2):213-24. doi: 10.1207/s15327914nc5202_11. Nutr Cancer. 2005. PMID: 16201852
Cited by
- Sulforaphane and related mustard oils in focus of cancer prevention and therapy.
Herr I, Lozanovski V, Houben P, Schemmer P, Büchler MW. Herr I, et al. Wien Med Wochenschr. 2013 Feb;163(3-4):80-8. doi: 10.1007/s10354-012-0163-3. Epub 2012 Dec 7. Wien Med Wochenschr. 2013. PMID: 23224634 - Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects.
Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Myzak MC, et al. Exp Biol Med (Maywood). 2007 Feb;232(2):227-34. Exp Biol Med (Maywood). 2007. PMID: 17259330 Free PMC article. - Sulforaphane has opposing effects on TNF-alpha stimulated and unstimulated synoviocytes.
Fragoulis A, Laufs J, Müller S, Soppa U, Siegl S, Reiss LK, Tohidnezhad M, Rosen C, Tenbrock K, Varoga D, Lippross S, Pufe T, Wruck CJ. Fragoulis A, et al. Arthritis Res Ther. 2012 Oct 27;14(5):R220. doi: 10.1186/ar4059. Arthritis Res Ther. 2012. PMID: 23072510 Free PMC article. - Impact of Epigenetic Dietary Components on Cancer through Histone Modifications.
Gao Y, Tollefsbol TO. Gao Y, et al. Curr Med Chem. 2015;22(17):2051-64. doi: 10.2174/0929867322666150420102641. Curr Med Chem. 2015. PMID: 25891109 Free PMC article. Review.
References
- Maheo K, Morel F, Langouet S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A. Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res. 1997;57:3649–3652. - PubMed
- Barcelo S, Gardiner JM, Gescher A, Chipman JK. CYP2E1-mediated mechanism of anti-genotoxicity of the broccoli constituent sulforaphane. Carcinogenesis. 1996;17:277–282. - PubMed
- Dashwood RH. Modulation of heterocyclic amine-induced mutagenicity and carcinogenicity: an ‘A-to-Z’ guide to chemopreventive agents, promoters, and transgenic models. Mutat Res. 2002;511:89–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA090890/CA/NCI NIH HHS/United States
- CA90890/CA/NCI NIH HHS/United States
- CA80176/CA/NCI NIH HHS/United States
- R01 CA065525/CA/NCI NIH HHS/United States
- CA66525/CA/NCI NIH HHS/United States
- P30 ES000210/ES/NIEHS NIH HHS/United States
- P30 ES00210/ES/NIEHS NIH HHS/United States
- R01 CA080176/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous