TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity? - PubMed (original) (raw)
TNFalpha-induced AMPA-receptor trafficking in CNS neurons; relevance to excitotoxicity?
Dmitri Leonoudakis et al. Neuron Glia Biol. 2004 Aug.
Abstract
Injury and disease in the CNS increases the amount of tumor necrosis factor alpha (TNFalpha) that neurons are exposed to. This cytokine is central to the inflammatory response that occurs after injury and during prolonged CNS disease, and contributes to the process of neuronal cell death. Previous studies have addressed how long-term apoptotic-signaling pathways that are initiated by TNFalpha might influence these processes, but the effects of inflammation on neurons and synaptic function in the timescale of minutes after exposure are largely unexplored. Our published studies examining the effect of TNFalpha on trafficking of AMPA-type glutamate receptors (AMPARs) in hippocampal neurons demonstrate that glial-derived TNFalpha causes a rapid (<15 minute) increase in the number of neuronal, surface-localized, synaptic AMPARs leading to an increase in synaptic strength. This indicates that TNFalpha-signal transduction acts to facilitate increased surface localization of AMPARs from internal postsynaptic stores. Importantly, an excess of surface localized AMPARs might predispose the neuron to glutamate-mediated excitotoxicity and excessive intracellular calcium concentrations, leading to cell death. This suggests a new mechanism for excitotoxic TNFalpha-induced neuronal death that is initiated minutes after neurons are exposed to the products of the inflammatory response. Here we review the importance of AMPAR trafficking in normal neuronal function and how abnormalities that are mediated by glial-derived cytokines such as TNFalpha can be central in causing neuronal disorders. We have further investigated the effects of TNFalpha on different neuronal cell types and present new data from cortical and hippocampal neurons in culture. Finally, we have expanded our investigation of the temporal profile of the action of this cytokine relevant to neuronal damage. We conclude that TNFalpha-mediated effects on AMPAR trafficking are common in diverse neuronal cell types and very rapid in their onset. The abnormal AMPAR trafficking elicited by TNFalpha might present a novel target to aid the development of new neuroprotective drugs.
Figures
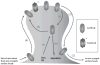
Fig. 1. Glutamate receptor trafficking in the dendritic spine during normal and abnormal synaptic function
AMPARs can be removed rapidly from the synapse on dendritic spines by either endocytosis (1) or lateral movement to extrasynaptic surface locales (2). Endocytosed AMPARs can be trafficked to lysosomes for degradation (6) or recycled back to the synapse (3). AMPARs can also be transported into the synapse after synthesis in either somatic or local dendritic endoplasmic reticulum (5) and then exocytosed to the synapse (3). Lateral movement of extrasynaptic receptors to the synapse (4) can also replenish receptors at this location. ⋆, recent work shows that NMDARs also are trafficked into and out of the synapse though possibly at a different timecourse than AMPARs (reviewed in Carroll and Zukin, 2002). The increase in surface receptors following either increased delivery via pathways 3 and 4 or a reduction in removal via pathways 1,6 and 2 might increase the possibility of excitotoxicity during acute trauma and neuronal overactivation during epilepsy.
Fig. 2. Perinuclear TNFα staining in astrocytes in culture
(A) Astrocytes stained for TNFα. Often, staining is punctate and perinuclear in appearance. Astrocyte cultures lacking neurons were grown as described (Beattie et al., 2002) and stained as described below. Co-staining with a GFAP-specific antibody (R&D Systems; Cat. No. AF-510-NA; 1μg ml−1) confirmed these cells as astrocytes (data not shown). (B) Neurons also frequently exhibit TNFα immunoreactivity that is more evenly distributed and less punctate than observed in astrocytes. Absence of co-staining with a GFAP-specific antbody (R&D Systems; Cat. No. AF-510-NA; 1 μg ml−1) confirmed these cells as neurons (data not shown). Staining for TNFα used a goat anti-TNFα antibody (R&D Systems; Cat. No. AF-510-NA; 1 μg ml−1) applied to fixed, permeablized cells for 1 hour at room temperature. Cells were then washed three times in blocking solution (PBS, 1% BSA, 0.8 μg ml−1 saponin). The signal was visualized by application of a donkey anti-goat antibody conjugated to Cy3 (Jackson ImmunoResearch), at a dilution of 1:800 for 45 minutes at room temperature. The cells were then washed three times in PBS and mounted.
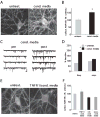
Fig. 3. Astrocyte-conditioned media increases surface expression of AMPARs and synaptic strength via TNFα
(A) Examples of surface AMPAR staining in untreated and conditioned-media-treated neurons. (B) Quantification of effects of conditioned media on surface AMPAR staining. (⋆P < 0.01; untreated 100 ± 9%, n = 45; conditioned media 152 ± 9%, n = 37). (C) Examples of miniature EPSCs before and after application of conditioned media (calibration bars, 20 pA, 500 mseconds). (D) Mean change in miniature EPSC frequency and amplitude in cells treated with either control or conditioned media. (n = 7, untreated; n = 8 conditioned-media-treated cells; ⋆P < 0.01; % initial mEPSC frequency: conditioned media 185 ± 25%; normal media 76 ± 5%; % initial mEPSC amplitude: conditioned media 117 ± 14%; normal media 96 ± 2%). (E) Examples of surface AMPAR staining in an untreated cell and a cell treated with conditioned media containing TNFR1. (F) Quantification of effects of conditioned media containing TNFR1, anti-TNFα antibody and the matrix metalloproteinase inhibitor GM6001. (n = 31–45 for each group; untreated 100 ± 9%; TNFR1 and conditioned media 113 ± 13%; anti-TNFα antibody and conditioned media 80 ± 10%; GM6001 and conditioned media 78 ± 9%). Figure reproduced, with permission, from Beattie et al. (2002).
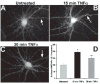
Fig. 4. TNFα increases surface expression of AMPARs in primary hippocampal neurons in culture
(A–C) Examples of surface AMPAR staining in untreated (A), and neurons treated with TNFα for 15 minutes (B) and 30 minutes (C). (D) Quantitation of the effects of TNFα on surface AMPAR staining (n = 50–70 for each group pooled from at least three different experiments; ⋆P = 0.08; ⋆⋆P = 0.4; untreated, 100 ± 14%; 15 minute TNFα, 195 ± 10%; 30 minute TNFα, 149 ± 10%). Arrows point to processes where very defined punctuate staining appears after TNF application (B,C). Experimental methods: After treatment of 17–25-day-old neuron cultures (see Beattie et al., 2002 for culture methods) with 6 nM TNFα at 37°C for the times indicated, neurons were chilled on ice, washed with cold PBS, and surface AMPARs visualized by indirect immunofluorescence using a rabbit antibody to the extracellular N-terminus of GluR1 (Ab-1%, Oncogene Research Products) at a dilution of 1:20 for 1 hour at 4°C. Neurons were then washed with cold PBS and fixed with 4% paraformaldehyde/4% sucrose in PBS. The non-permeablized cells were then blocked with 3% BSA, 1% goat serum in PBS and a donkey anti-rabbit secondary Ab conjugated to Alexafluor 568 (Molecular Probes) was applied at a dilution of 1:800 for 45 minutes at room temperature, followed by thorough washing with PBS and mounting on slides with Fluoromount G (Electron Microscopy Services). Neurons were visualized and images captured with immunofluorescence microscopy as described in Beattie et al. (2002). For individual experiments, images for all conditions were analyzed using identical acquisition parameters and untreated and treated cells from the same culture preparation compared. Images from each experiment were obtained using a threshold equal to the average background fluorescence in untreated, control cells. The total area of fluorescently labeled surface AMPARs was measured automatically by Metamorph software and divided by the total cell area (determined using a lower threshold level to measure background fluorescence produced by fixed cells). For each experiment, the fluorescence of all cells was normalized by dividing by the average fluorescence of the untreated control cells. Each experimental manipulation was repeated a minimum of three times using different culture preparations. n represents the number of microscope fields examined. Statistical significance between individual experimental groups and the control group was determined using Student’s _t_-test. Error bars represent s.e.m.
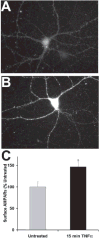
Fig. 5. TNFα rapidly increases surface expression of AMPARs in primary cortical neurons in culture
(A–B) Examples of surface AMPAR staining in untreated (A) and cortical neurons treated with TNFα for 15 minutes (B). (C) Quantification of effects of TNFα on surface AMPAR staining (n = 70 for each group pooled from four experiments; ⋆P = 0.01; untreated, 100 ± 11%; 15 minute TNFα, 147 ± 11%). Cortical neurons were prepared, treated and visualized as described in Fig. 4.
Similar articles
- Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane.
Ferguson AR, Christensen RN, Gensel JC, Miller BA, Sun F, Beattie EC, Bresnahan JC, Beattie MS. Ferguson AR, et al. J Neurosci. 2008 Oct 29;28(44):11391-400. doi: 10.1523/JNEUROSCI.3708-08.2008. J Neurosci. 2008. PMID: 18971481 Free PMC article. - Rapid tumor necrosis factor alpha-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excitotoxicity.
Leonoudakis D, Zhao P, Beattie EC. Leonoudakis D, et al. J Neurosci. 2008 Feb 27;28(9):2119-30. doi: 10.1523/JNEUROSCI.5159-07.2008. J Neurosci. 2008. PMID: 18305246 Free PMC article. - Cannabinoid receptor activation reduces TNFalpha-induced surface localization of AMPAR-type glutamate receptors and excitotoxicity.
Zhao P, Leonoudakis D, Abood ME, Beattie EC. Zhao P, et al. Neuropharmacology. 2010 Feb;58(2):551-8. doi: 10.1016/j.neuropharm.2009.07.035. Epub 2009 Aug 4. Neuropharmacology. 2010. PMID: 19654014 Free PMC article. - Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.
Levite M. Levite M. J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review. - The AMPAR subunit GluR2: still front and center-stage.
Tanaka H, Grooms SY, Bennett MV, Zukin RS. Tanaka H, et al. Brain Res. 2000 Dec 15;886(1-2):190-207. doi: 10.1016/s0006-8993(00)02951-6. Brain Res. 2000. PMID: 11119696 Review.
Cited by
- Role of Aβ in Alzheimer's-related synaptic dysfunction.
Zhang H, Jiang X, Ma L, Wei W, Li Z, Chang S, Wen J, Sun J, Li H. Zhang H, et al. Front Cell Dev Biol. 2022 Aug 26;10:964075. doi: 10.3389/fcell.2022.964075. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092715 Free PMC article. Review. - Genetic deletion of TNF receptor suppresses excitatory synaptic transmission via reducing AMPA receptor synaptic localization in cortical neurons.
He P, Liu Q, Wu J, Shen Y. He P, et al. FASEB J. 2012 Jan;26(1):334-45. doi: 10.1096/fj.11-192716. Epub 2011 Oct 7. FASEB J. 2012. PMID: 21982949 Free PMC article. - Oligodendrocytes in central nervous system diseases: the effect of cytokine regulation.
Zhang C, Qiu M, Fu H. Zhang C, et al. Neural Regen Res. 2024 Oct 1;19(10):2132-2143. doi: 10.4103/1673-5374.392854. Epub 2024 Jan 8. Neural Regen Res. 2024. PMID: 38488548 Free PMC article. - Altered presymptomatic AMPA and cannabinoid receptor trafficking in motor neurons of ALS model mice: implications for excitotoxicity.
Zhao P, Ignacio S, Beattie EC, Abood ME. Zhao P, et al. Eur J Neurosci. 2008 Feb;27(3):572-9. doi: 10.1111/j.1460-9568.2008.06041.x. Eur J Neurosci. 2008. PMID: 18279310 Free PMC article. - Isolated corpus callosum lesion associated with cytokine storm in COVID-19.
Arıkan FA, Akdağ G, Çetiner M, Uysal N, Kabay SC. Arıkan FA, et al. Proc (Bayl Univ Med Cent). 2022 Mar 9;35(3):337-338. doi: 10.1080/08998280.2022.2044655. eCollection 2022. Proc (Bayl Univ Med Cent). 2022. PMID: 35518813 Free PMC article.
References
- Allan SM. Varied actions of proinflammatory cytokines on excitotoxic cell death in the rat central nervous system. Journal of Neuroscience Research. 2002;67:428–434. - PubMed
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nature Reviews Neuroscience. 2001;2:734–744. - PubMed
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. Journal of Neurochemistry. 2001;76:846–854. - PubMed
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature Neuroscience. 2000;3:1291–1300. - PubMed
LinkOut - more resources
Full Text Sources