The effects of sex and hormonal status on restraint-stress-induced working memory impairment - PubMed (original) (raw)
The effects of sex and hormonal status on restraint-stress-induced working memory impairment
Rebecca M Shansky et al. Behav Brain Funct. 2006.
Abstract
Background: Restraint stress has been shown to elicit numerous effects on hippocampal function and neuronal morphology, as well as to induce dendritic remodeling in the prefrontal cortex (PFC). However, the effects of acute restraint stress on PFC cognitive function have not been investigated, despite substantial evidence that the PFC malfunctions in many stress-related disorders.
Methods: The present study examined the effects of restraint stress on PFC function in both male rats and cycling female rats in either the proestrus (high estrogen) or estrus (low estrogen) phase of the estrus cycle. Animals were restrained for 60 or 120 minutes and then tested on spatial delayed alternation, a PFC-mediated task. Performance after stress was compared to performance on a different day under no-stress conditions, and analyzed using analysis of variance (ANOVA).
Results: Sixty minutes of restraint impaired only females in proestrus, while 120 minutes of restraint produced significant impairments in all animals. Increases in task completion times did not affect performance.
Conclusion: These results demonstrate an interaction between hormonal status and cognitive response to stress in female rats, with high estrogen levels being associated with amplified sensitivity to stress. This effect has been previously observed after administration of a pharmacological stressor (the benzodiazepine inverse agonist FG7142), and results from both studies may be relevant to the increased prevalence of stress-related disorders, such as major depressive disorder, in cycling women. Overall, the results show that restraint stress has important effects on the cognitive functions of the PFC, and that hormonal influences in the PFC are an important area for future research.
Figures
Figure 1
Representative Training Schedule. Rats were tested daily, Monday through Friday. In order to be eligible for stress testing, three criteria must be met: 1) The animal must have achieved a score of 6, 7 or 8 (out of 10) for at least two consutive days; 2) at least one week must have passed since the last stress exposure; 3) females must be cycling regularly on a 4–5 day cycle, and it must be anticipated that they will be in either proestrus or estrus on stress day. In this figure, the rat is ready for testing on the first Wednesday because her two previous scores are 7 and 8, and she will be in estrus. In the sond week, she does not receive stress treatment because her scores do not qualify her on a day when she will be in estrus or proestrus. In the third week, her scores on Tuesday and Wednesday qualify her for treatment on Thursday, and she will be in proestrus, so she receives treatment. In week four, her scores and cycle qualify her for treatment on Friday.
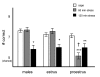
Figure 2
Females in proestrus are more sensitive to the PFC-impairing effects of stress than males or females in estrus. Animals were restrained for 0, 60 or 120 min prior to testing on the delayed alternation T-maze task. Results are represented as mean +/- SEM number correct. Mean scores after 0, 60 and 120 min restraint were 7.4 +/- .22, 7.6 +/- .3 and 5.7 +/- .51 for males; 7.1 +/- .18, 7.4 +/- .4 and 5.9 +/- .51 for females in estrus; and 6.9 +/- .2, 5 +/- .35 and 5.9 +/- .5 for females in proestrus. * = significantly different from self in control conditions, p < .05, ** = p < .005, *** = p < .0005, † = significantly different from self during estrus, p < .02.
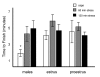
Figure 3
Group differences in time were observed, but they did not correlate with differences in performance. Task completion times were recorded for all groups. Results are represented as mean +/- SEM minutes to finish. Mean task completion times (in minutes) after 0, 60 and 120 min were 1.8 +/- .4, 4.5 +/- 1.1 and 5 +/- 1 for males; 4.3 +/- .5, 5.8 +/- .7 and 4.8 +/- .64 for females in estrus; and 4.7 +/- 1.6, 5.3 +/- .6 and 3.9 +/- .4 for females in proestrus. * = significantly different from all other groups.
Similar articles
- Estrogen prevents norepinephrine alpha-2a receptor reversal of stress-induced working memory impairment.
Shansky RM, Bender G, Arnsten AF. Shansky RM, et al. Stress. 2009 Sep;12(5):457-63. doi: 10.1080/10253890802520988. Stress. 2009. PMID: 19005873 Free PMC article. - Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction.
Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AF. Shansky RM, et al. Mol Psychiatry. 2004 May;9(5):531-8. doi: 10.1038/sj.mp.4001435. Mol Psychiatry. 2004. PMID: 14569273 - A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex.
Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. Birnbaum S, et al. Biol Psychiatry. 1999 Nov 1;46(9):1266-74. doi: 10.1016/s0006-3223(99)00138-9. Biol Psychiatry. 1999. PMID: 10560032 - Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle.
Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Conrad CD, et al. Pharmacol Biochem Behav. 2004 Jul;78(3):569-79. doi: 10.1016/j.pbb.2004.04.025. Pharmacol Biochem Behav. 2004. PMID: 15251266 - Sex differences in chronic stress effects on memory in rats.
Luine V. Luine V. Stress. 2002 Sep;5(3):205-16. doi: 10.1080/1025389021000010549. Stress. 2002. PMID: 12186683 Review.
Cited by
- Sex-specific impairment and recovery of spatial learning following the end of chronic unpredictable restraint stress: potential relevance of limbic GAD.
Ortiz JB, Taylor SB, Hoffman AN, Campbell AN, Lucas LR, Conrad CD. Ortiz JB, et al. Behav Brain Res. 2015 Apr 1;282:176-84. doi: 10.1016/j.bbr.2014.12.051. Epub 2015 Jan 13. Behav Brain Res. 2015. PMID: 25591480 Free PMC article. - Kappa opioid receptor antagonism protects working memory performance from mild stress exposure in Rhesus macaques.
Wallace TL, Martin WJ, Arnsten AFT. Wallace TL, et al. Neurobiol Stress. 2022 Sep 24;21:100493. doi: 10.1016/j.ynstr.2022.100493. eCollection 2022 Nov. Neurobiol Stress. 2022. PMID: 36532373 Free PMC article. - The effect of childhood trauma on spatial cognition in adults: a possible role of sex.
Syal S, Ipser J, Phillips N, Thomas KG, van der Honk J, Stein DJ. Syal S, et al. Metab Brain Dis. 2014 Jun;29(2):301-10. doi: 10.1007/s11011-014-9497-4. Epub 2014 Feb 21. Metab Brain Dis. 2014. PMID: 24553877 - Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress.
Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Hains AB, et al. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17957-62. doi: 10.1073/pnas.0908563106. Epub 2009 Sep 11. Proc Natl Acad Sci U S A. 2009. PMID: 19805148 Free PMC article. - Estrogen prevents norepinephrine alpha-2a receptor reversal of stress-induced working memory impairment.
Shansky RM, Bender G, Arnsten AF. Shansky RM, et al. Stress. 2009 Sep;12(5):457-63. doi: 10.1080/10253890802520988. Stress. 2009. PMID: 19005873 Free PMC article.
References
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous