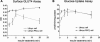Protein kinase Czeta mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells - PubMed (original) (raw)
Protein kinase Czeta mediates insulin-induced glucose transport through actin remodeling in L6 muscle cells
Li-Zhong Liu et al. Mol Biol Cell. 2006 May.
Abstract
Protein kinase C (PKC) zeta has been implicated in insulin-induced glucose uptake in skeletal muscle cell, although the underlying mechanism remains unknown. In this study, we investigated the effect of PKCzeta on actin remodeling and glucose transport in differentiated rat L6 muscle cells expressing myc-tagged glucose transporter 4 (GLUT4). On insulin stimulation, PKCzeta translocated from low-density microsomes to plasma membrane accompanied by increase in GLUT4 translocation and glucose uptake. Z-scan confocal microscopy revealed a spatial colocalization of relocated PKCzeta with the small GTPase Rac-1, actin, and GLUT4 after insulin stimulation. The insulin-mediated colocalization, PKCzeta distribution, GLUT4 translocation, and glucose uptake were inhibited by wortmannin and cell-permeable PKCzeta pseudosubstrate peptide. In stable transfected cells, overexpression of PKCzeta caused an insulin-like effect on actin remodeling accompanied by a 2.1-fold increase in GLUT4 translocation and 1.7-fold increase in glucose uptake in the absence of insulin. The effects of PKCzeta overexpression were abolished by cell-permeable PKCzeta pseudosubstrate peptide, but not wortmannin. Transient transfection of constitutively active Rac-1 recruited PKCzeta to new structures resembling actin remodeling, whereas dominant negative Rac-1 prevented the insulin-mediated PKCzeta translocation. Together, these results suggest that PKCzeta mediates insulin effect on glucose transport through actin remodeling in muscle cells.
Figures
Figure 1.
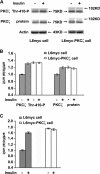
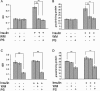
Insulin-mediated PKCζ phosphorylation, expression, and activity. L6myc and L6myc-PKCζ myotubes were treated with or without 100 nmol/l insulin for 5 min at 37°C, and then with 15 μg of whole cell lysates were subjected to 10% SDS-PAGE and immunoblotted with indicated antibodies (anti-phospho-PKCλ/ζ for PKCζ activity, anti-PKC λ/ζ for PKCζ protein, and anti-actin for actin protein). The same membranes were first blotted for phosphorylation followed by stripping and reblotting for protein. The EGFP tag ensured transfected PKCζ protein running ∼27 kDa higher than the endogenous PKCζ, ∼75 kDa indicated by arrowhead (A). The amounts of phosphorylated PKCζ and protein kinase Cζ protein were quantified by densitometric scanning and analyzed with Bio-Rad Molecular Analysis software. Pixel intensity was normalized, and a value of 1 was assigned to the basal condition (B). Insulin-induced PKCζ activity was measured with specific immunoprecipitates from the 30 μg of whole cell lysates, as described in Materials and Methods (C). The data are representative of three experiments.
Figure 2.
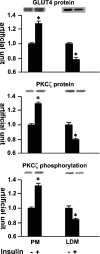
Insulin-mediated protein kinase Cζ and GLUT4 translocation shown by cell fractionation. Serum-depleted (3 h) L6myc cells were treated with or without 100 nmol/l insulin for 5 min. Subcellular fractionation was performed to purify the PM and LDM fractions as described in Materials and Methods. Equal amount proteins (20 μg) were subjected to 10% SDS-PAGE to immunoblot for GLUT4myc and PKCζ phosphorylation with corresponding antibodies first (anti-phospho-PKCλ/ζ for PKCζ phosphorylation, and anti-myc for GLUT4myc protein), after stripping, the same membrane (measured for protein kinase Cζ phosphorylation) was reblotted for PKCζ protein (anti-PKCλ/ζ for PKCζ protein) after stripping as described in Materials and Methods. The amount of phosphorylated PKCζ, PKCζ protein, and GLUT4myc were quantified by densitometric scanning and analysis with Bio-Rad Molecular Analysis software. Pixel intensity was normalized, and a value of 1 was assigned to the basal condition. The data are representative of three experiments as the mean ± SE and *p < 0.01 compared with basal state.
Figure 3.
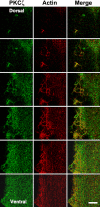
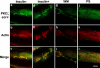
Insulin-mediated colocalization of PKCζ with actin shown by laser confocal microscopy. L6myc cells were serum starved 3 h and then treated with 100 nmol/l insulin for 5 min at 37°C. Afterward, the cells were fixed, permeabilized, and double stained for PKCζ (anti-PKCζ antibody followed by Alexa 488 green-conjugated second antibody) and actin (Alexa red-phalloidin) as described in Materials and Methods. Serial optical sections of 0.5 μm thickness along the _z_-axis were generated as described in Materials and Methods. Optical slices taken from the dorsal to the ventral surface of myotubes show filamentous PKCζ (green) and actin (red) after 5 min of 100 nmol/l insulin treatment. The right line shows the merge of PKCζ and actin. The progression of images is linear from left to right and from top to bottom. Bar, 10 μm. The images are representative of five experiments.
Figure 4.
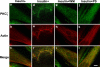
Inhibition effect of WM and PKCζ PS on insulin-mediated colocalization of PKCζ with actin remodeling in L6myc cells shown by laser confocal microscopy. Three hour-serum-starved L6myc cells were left untreated (a-c), or treated with 100 nmol/l insulin (d-f) for 5 min at 37°C, pretreated with 100 nmol/l WM (g-i) for 25 min, or 5 μmol/l PS (j-l) for 55 min at 37°C, followed by adding 100 nmol/l insulin and incubated at 37°C for 5 min. Then, the cells were fixed, permeabilized, stained, and scanned as described as Figure 3. Bar, 10 μm. The images are representative of five experiments.
Figure 5.
PKCζ-mediated insulin effect on actin remodeling and the inhibition effect of WM and PKCζ PS in L6myc PKCζ cells shown by laser confocal microscopy. L6myc-protein kinase Cζ cells were serum starved 3 h and were left untreated (a-c), or treated with 100 nmol/l insulin for 5 min at 37°C (d-f), preincubated with 100 nmol/l WM (g-i) for 25 min, or 5 μmol/l PS (j-l) for 55 min at 37°C, followed by adding 100 nmol/l insulin and incubated at 37°C for 5 min. Then, the cells were fixed, permeabilized, stained, and scanned as described as Figure 3. Because the EGFP-tagged PKCζ gave the auto-green fluorescence in L6myc-PKCζ cells, Alexa red-phalloidin was used for illustrating the F-actin. Bar, 10 μm. The images are representative of five experiments.
Figure 6.
PKCζ-mediated insulin effect on surface GLUT4 translocation and glucose uptake. L6myc cells and L6myc PKCζ cells were prepared on 24-well plates or 12-well plates for measurement of surface GLUT4 (A) or glucose uptake (B) as described in Materials and Methods. L6myc and L6myc-PKCζ cells were treated for the indicated times with 100 nmol/l insulin at 37°C. Cell surface density of GLUT4myc and 2-deoxyglucose uptake was measured in parallel culture plates. Data points are the mean ± SE of five to eight experiments performed in triplicate. Insulin-stimulated GLUT4myc translocation and glucose uptake were expressed as optical density (OD) and picomoles per minute per milligram of protein separately.
Figure 7.
Inhibition effects of WM and PKCζ PS on GLUT4 translocation and glucose uptake in L6myc cells and L6myc PKCζ cells. L6myc cells (A and B) and L6myc PKCζ cells (C and D) were prepared on 24-well plates or 12-well plates for measurement of surface GLUT4 (A and C) and glucose uptake (B and D) as described in Materials and Methods. For insulin stimulation groups, cells were treated with 100 nmol/l insulin for 5 min at 37°C; for the inhibition groups, cells were first treated with 100 nmol/l wortmannin for 25 min or 5 μmol/l PS for 55 min, followed by adding 100 nmol/l insulin and incubated at 37°C for 5 min. Cell surface density of GLUT4myc (A and C) and 2-deoxyglucose uptake (B and D) were measured in parallel culture plates. Data points are the mean ± SE of four experiments performed in triplicate. Insulin-stimulated GLUT4myc translocation and glucose uptake are expressed as OD and picomoles per minute per milligram of protein. **p < 0.01.
Figure 8.
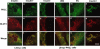
Association of protein kinase Cζ with GLUT4 and the inhibition effect of WM and PKCζ PS by laser confocal microscopy. L6myc cells (a-f) and L6myc PKCζ cells (g-r) were serum starved 3 h and were left untreated (a-c, g-i), or treated with 100 nmol/l insulin (d-f, p-r) for 5 min at 37°C, 100 nmol/l WM (i and j) for 25 min, or 5 μmol/l PS (m-o) for 55 min at 37°C. Afterward, the cells were fixed, permeabilized, and double stained for PKCζ (anti-PKCζ antibody followed by Alexa 488 green-conjugated second antibody) and GLUT4myc (anti-myc antibody followed by Alexa 546 red-conjugated second antibody) as described in Materials and Methods. Cells were scanned along the _z_-axis. Bar, 10 μm. The images are representative of five experiments.
Figure 9.
Interaction of PKCζ with Rac-1 on actin remodeling shown by laser confocal microscopy. L6myc myoblasts were prepared in medium containing 2% (vol/vol) FBS on six-well plates with coverslips. Plasmids contained HA-tagged Rac-1 (CA or DN) gene were introduced to the cells with Lipofectmine 2000 at the start of day 4 after seeding, and cells were maintained for another 72 h. Then, cells were serum-starved 3 h and stimulated with or without 100 nmol/l insulin for 5 min at 37°C. Afterward, the cells were fixed, permeabilized, and double stained for endogenous PKCζ (anti-PKCζ antibody followed by Alexa 488 green-conjugated second antibody; a, d, g, and j), endogenous Rac-1 (anti-Rac-1 antibody followed by Alexa 546 red-conjugated second antibody; b and e), or HA-tag for transfected Rac-1 CA (h) and Rac-1 DN (k) (anti-HA antibody followed by Alexa 546 red-conjugated second antibody) as described in Materials and Methods. Serial optical sections of 0.5 μm thickness along the _z_-axis were generated as described in Materials and Methods. Bar, 10 μm. The images are representative of three experiments.
Similar articles
- Protein kinase C-zeta regulation of GLUT4 translocation through actin remodeling in CHO cells.
Liu XJ, Yang C, Gupta N, Zuo J, Chang YS, Fang FD. Liu XJ, et al. J Mol Med (Berl). 2007 Aug;85(8):851-61. doi: 10.1007/s00109-007-0232-z. Epub 2007 Jul 10. J Mol Med (Berl). 2007. PMID: 17619838 - Identification of 80K-H as a protein involved in GLUT4 vesicle trafficking.
Hodgkinson CP, Mander A, Sale GJ. Hodgkinson CP, et al. Biochem J. 2005 Jun 15;388(Pt 3):785-93. doi: 10.1042/BJ20041845. Biochem J. 2005. PMID: 15707389 Free PMC article. - Fatty acids inhibit insulin-mediated glucose transport associated with actin remodeling in rat L6 muscle cells.
Zhao HL, Liu LZ, Sui Y, Ho SK, Tam SK, Lai FM, Chan JC, Tong PC. Zhao HL, et al. Acta Diabetol. 2010 Dec;47(4):331-9. doi: 10.1007/s00592-010-0225-1. Epub 2010 Sep 17. Acta Diabetol. 2010. PMID: 20848165 - Rac1 signalling towards GLUT4/glucose uptake in skeletal muscle.
Chiu TT, Jensen TE, Sylow L, Richter EA, Klip A. Chiu TT, et al. Cell Signal. 2011 Oct;23(10):1546-54. doi: 10.1016/j.cellsig.2011.05.022. Epub 2011 Jun 13. Cell Signal. 2011. PMID: 21683139 Review. - Protein kinase Czeta and glucose uptake.
Liu LZ, He AB, Liu XJ, Li Y, Chang YS, Fang FD. Liu LZ, et al. Biochemistry (Mosc). 2006 Jul;71(7):701-6. doi: 10.1134/s0006297906070017. Biochemistry (Mosc). 2006. PMID: 16903823 Review.
Cited by
- The activated CD36-Src axis promotes lung adenocarcinoma cell proliferation and actin remodeling-involved metastasis in high-fat environment.
Liu LZ, Wang B, Zhang R, Wu Z, Huang Y, Zhang X, Zhou J, Yi J, Shen J, Li MY, Dong M. Liu LZ, et al. Cell Death Dis. 2023 Aug 23;14(8):548. doi: 10.1038/s41419-023-06078-3. Cell Death Dis. 2023. PMID: 37612265 Free PMC article. - MYH10 Governs Adipocyte Function and Adipogenesis through Its Interaction with GLUT4.
Kislev N, Mor-Yossef Moldovan L, Barak R, Egozi M, Benayahu D. Kislev N, et al. Int J Mol Sci. 2022 Feb 21;23(4):2367. doi: 10.3390/ijms23042367. Int J Mol Sci. 2022. PMID: 35216482 Free PMC article. - Lipid oversupply induces CD36 sarcolemmal translocation via dual modulation of PKCζ and TBC1D1: an early event prior to insulin resistance.
Zhu B, Li MY, Lin Q, Liang Z, Xin Q, Wang M, He Z, Wang X, Wu X, Chen GG, Tong PC, Zhang W, Liu LZ. Zhu B, et al. Theranostics. 2020 Jan 1;10(3):1332-1354. doi: 10.7150/thno.40021. eCollection 2020. Theranostics. 2020. PMID: 31938068 Free PMC article. - Combined Hyperglycemia- and Hyperinsulinemia-Induced Insulin Resistance in Adipocytes Is Associated With Dual Signaling Defects Mediated by PKC-ζ.
Lu H, Bogdanovic E, Yu Z, Cho C, Liu L, Ho K, Guo J, Yeung LSN, Lehmann R, Hundal HS, Giacca A, Fantus IG. Lu H, et al. Endocrinology. 2018 Apr 1;159(4):1658-1677. doi: 10.1210/en.2017-00312. Endocrinology. 2018. PMID: 29370351 Free PMC article. - The Effect of SIN1 and Microtubules on Insulin Induced PKC ζ Activation.
Yingying X, Caijuan W, Yenan Y, Yuqin T, Xueqin C, Zhongming W. Yingying X, et al. Med Sci Monit. 2017 Jul 28;23:3666-3672. doi: 10.12659/msm.905555. Med Sci Monit. 2017. PMID: 28751630 Free PMC article.
References
- Bandyopadhyay, G., Sajan, M. P., Kanoh, Y., Standaert, M. L., Quon, M. J., Lea-Currie, R., Sen, A., and Farese, R. V. (2002). Protein kinase C-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. J. Clin. Endocrinol. Metab. 87, 716-723. - PubMed
- Bandyopadhyay, G., Standaert, M. L., Galloway, L., Moscat, J., and Farese, R. V. (1997a). Evidence for involvement of protein kinase C-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology 138, 4721-4731. - PubMed
- Bandyopadhyay, G., Standaert, M. L., Kikkawa, U., Ono, Y., Moscat, J., and Farese, R. V. (1999). Effects of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinases C-zeta and C-lambda. Biochem. J. 337, 461-470. - PMC - PubMed
- Bandyopadhyay, G., Standaert, M. L., Zhao, L., Yu, B., Avignon, A., Galloway, L., Karnam, P., Moscat, J., and Farese, R. V. (1997b). Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for protein kinase C-zeta in glucose transport. J. Biol. Chem. 272, 2551-2558. - PubMed
- Beeson, M., et al. (2003). Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes 52, 1926-1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous