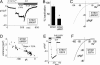STIM1 has a plasma membrane role in the activation of store-operated Ca(2+) channels - PubMed (original) (raw)
STIM1 has a plasma membrane role in the activation of store-operated Ca(2+) channels
Maria A Spassova et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Receptor-induced Ca(2+) signals are key to the function of all cells and involve release of Ca(2+) from endoplasmic reticulum (ER) stores, triggering Ca(2+) entry through plasma membrane (PM) "store-operated channels" (SOCs). The identity of SOCs and their coupling to store depletion remain molecular and mechanistic mysteries. The single transmembrane-spanning Ca(2+)-binding protein, STIM1, is necessary in this coupling process and is proposed to function as an ER Ca(2+) sensor to provide the trigger for SOC activation. Here we reveal that, in addition to being an ER Ca(2+) sensor, STIM1 functions within the PM to control operation of the Ca(2+) entry channel itself. Increased expression levels of STIM1 correlate with a gain in function of Ca(2+) release-activated Ca(2+) (CRAC) channel activity. Point mutation of the N-terminal EF hand transforms the CRAC channel current (I(CRAC)) into a constitutively active, Ca(2+) store-independent mode. Mutants in the EF hand and cytoplasmic C terminus of STIM1 alter operational parameters of CRAC channels, including pharmacological profile and inactivation properties. Last, Ab externally applied to the STIM1 N-terminal EF hand blocks both I(CRAC) in hematopoietic cells and SOC-mediated Ca(2+) entry in HEK293 cells, revealing that STIM1 has an important functional presence within the PM. The results reveal that, in addition to being an ER Ca(2+) sensor, STIM1 functions within the PM to exert control over the operation of SOCs. As a cell surface signaling protein, STIM1 represents a key pharmacological target to control fundamental Ca(2+)-regulated processes including secretion, contraction, metabolism, cell division, and apoptosis.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
CRAC channel function correlates with altered expression of STIM1. (A) Development of _I_CRAC in Jurkat T cells overexpressing either STIM1 (red) or control empty vector (black). Current at −80 mV or +80 mV (red open circles) was measured in 20 mM extracellular Ca2+; addition of 50 μM 2-APB is indicated by the bar. (B) I_–_V profile determined at the time of maximal current in A. (C) Average maximal current at −100 mV in STIM1-overexpressing cells (n = 3) and control-expressing Jurkat T cells (n = 3). (D) Western analysis comparing STIM1 expression levels in RBL cells after knockdown and reexpression of STIM1. (E). The I_–_V profile for _I_CRAC was determined in DVF after maximal activation in Ca2+ex in control (blue), rSTIM1 knockdown (black), or rSTIM1 knockdown/hSTIM1-reexpressing RBL (red) cells. (Inset) Inactivation with overexpression of STIM1 WT in DVF. (F) Comparison of the average maximal current in DVF for control (n = 3), rSTIM1 knockdown (n = 3), and hSTIM1-reexpressing RBL (n = 4) cells. (G) _I_CRAC measured in rSTIM1 knockdown RBL cells reexpressing exogenous hSTIM1. Fifty micromolar 2-APB, but not 5 μM 2-APB, blocks the current in DVF. (H) Nonstationary noise analysis of the current in DVF from G to determine single-channel conductance (see Materials and Methods).
Fig. 2.
Expression of the E87A STIM1 mutant with decreased EF-hand Ca2+-binding affinity leads to constitutively active _I_CRAC in RBL cells. (A) Predicted 3D structure of STIM1 (57–591) generated by using
phyre
software (
). The domain structures shown, EF-hand, sterile-α motif (SAM), transmembrane-spanning region, coiled-coil regions, and proline-rich N terminus, were determined by similarity to proteins with known structure. (B) Either WT hSTIM1 or the E87A mutant with predicted lowered EF-hand Ca2+ affinity were reexpressed in rSTIM1 knockdown RBL cells. In contrast to the slow-developing store depletion-dependent current observed with STIM1 WT (black circles), _I_CRAC was constitutively active at the time of break-in (_t_o) with STIM1 E87A-reexpressing cells (−80 mV, red closed circles; +80 mV, red open circles). (C) I_–_V profiles of STIM1 WT or STIM1 E87A measured at the time points indicated by a, b, and c in B are similar to endogenous _I_CRAC, with high positive reversal potential in 10 mM extracellular Ca2+, reflecting Ca2+ selectivity. (D) Comparisons of average current observed at _t_o reveal significant differences between STIM1 WT(n = 3) and STIM1 E87A (n = 3), but not in maximal activation (_t_max).
Fig. 3.
Expression of STIM1 mutants alters the pharmacology and inactivation properties of _I_CRAC. (A) Measured at a holding potential of −80 mV, _I_CRAC failed to inactivate in DVF in Jurkat T cells overexpressing the STIM1-ΔM597 deletion mutant, as distinct from endogenous I_CRAC (see Fig. 4_A Inset) or I_CRAC measured after overexpression of hSTIM1 in RBL cells (Fig. 1_G). In contrast, other I_CRAC properties were similar to endogenous current: activation with passive Ca2+ store depletion, potentiation by 5 μM 2-APB, and inhibition by 50 μM 2-APB. (B) One minute after exposure to DVF, which followed maximal CRAC activation in 20 mM extracellular Ca2+, I_CRAC showed ≈25% inactivation when STIM1-ΔM597 was overexpressed (n = 6), significantly different from the ≈80% inactivation observed in STIM1 WT-overexpressing cells (n = 3; P < 0.05). (C) The I_–_V curve for I_CRAC in DVF was unaltered by overexpression of STIM1-ΔM597 (compared with STIM1 WT; Fig. 1_E). (D) Assessment of the single-channel conductance by noise analysis (as in Fig. 1_H) at −100 mV during inhibition by 2-APB in A revealed no differences from the single-channel current measured for STIM1 WT (Fig. 1_H). (E) hSTIM1 E87A mutant was overexpressed after knockdown of rSTIM1 in RBL cells. Sensitivity of _I_CRAC to 2-APB was greatly altered: _I_CRAC was immediately inhibited by 5 μM 2-APB, as opposed to the normal potentiation seen in A. (F) Overexpression of STIM1 E87A did not result in any changes in the I_–_V curve of I_CRAC in DVF as compared to STIM1 WT (see Fig. 1_E).
Fig. 4.
_I_CRAC and store-operated Ca2+ entry were inhibited by an anti-N-terminal STIM1 (25–139) mAb. Ab is depicted in red, and control (with or without isotype Ab) is depicted in black. (A_–_J) Jurkat T cells (A and B), HEK293 cells (C and D), or rSTIM1 knockdown RBL cells reexpressing hSTIM1 E87A mutant (F_–_K) were incubated for 30–60 min with STIM1 mAb or IgG2a isotype control Ab. (A) I_–_V curves for _I_CRAC were measured in DVF after maximal activation in Ca2+ in the presence or absence of 20 μg/ml mAb. Current inhibited by mAb was similar to control with respect to inward rectification and reversal potential. (Inset) Time course of _I_CRAC at −80 mV, measured after maximal activation. (B) Average maximal current measured at −100 mV was decreased by ≈70% in the presence of 20 μg/ml mAb compared with control (n = 3; P < 0.05). (C) SOC-mediated Ca2+ entry assessed in fura-2/acetoxymethyl ester-loaded HEK293 cells was similarly inhibited by the same mAb. No Ca2+ entry was observed with transient Ca2+ addition before 2 μM TG addition. Addition of Ca2+ after TG-induced store depletion revealed a reduction in SOC-induced Ca2+ entry in mAb-pretreated cells. (D) The effect of mAb on SOC was dose-dependent: ≈45% (P < 0.05) reduction at 5 μg/ml and ≈60% (P < 0.01) reduction at 10 μg/ml. (E) Western analysis of Jurkat T cell lysate using the same STIM1 mAb revealed only a single major band corresponding to the STIM1 protein. Lanes: 1, Size standards (kDa); 2, Jurkat T cell lysate. (F_–_K) _I_CRAC in hSTIM1 E87A-reexpressing RBL cells after rSTIM1 knockdown was inhibited by STIM1 Ab. (F) Incubation in isotype control Ab did not induce a change in I_CRAC compared with control cells (Fig. 3_E). (G) Incubation with mAb reduced _I_CRAC. Subsequent addition of 5 μM 2-APB resulted in complete blockade of the remaining current. (H) I_–_V curves of the cells shown in F and G in DVF were similar in inward rectification and reversal potential. (I) Average maximal current measured at −100 mV was decreased by ≈80% in the presence of mAb (n = 3; P < 0.05). (J) Noise analysis of the cell in G showed a preserved single-channel current of 16 fA, typical for ICRAC. (K) No change in the single-channel conductance was observed for the residual current after the mAb block (n = 3), compared with the isotype control (n = 3).
Fig. 5.
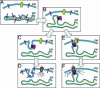
Models for the proposed actions of STIM1 in the activation of SOCs. (A) Cell at rest with ER stores filled with Ca2+. STIM1 molecules (pink) with Ca2+ bound are shown predominantly in the ER but also appear in the PM. The SOC (yellow) is closed. (B) Upon Ca2+ depletion of the ER, the ER STIM1 proteins become aggregated into puncta, shown as distinct regions of the ER close to the PM. (C) In the “insertional” model, the STIM1 protein is translocated and inserted into the PM. (D) The high content of STIM1 in the PM is sufficient to activate SOCs; this is shown as a hypothetical interaction between STIM1 and SOCs. (E) In the “influence” model, the aggregated STIM1 in puncta near the PM conformationally couples to STIM1 preexisting in the PM and causes association and reorganization of STIM1 in the PM. (F) This reorganization induces formation of a similar hypothetical complex between STIM1 and SOCs, leading again to channel activation.
Similar articles
- Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation.
Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Luik RM, et al. Nature. 2008 Jul 24;454(7203):538-42. doi: 10.1038/nature07065. Epub 2008 Jul 2. Nature. 2008. PMID: 18596693 Free PMC article. - A plasma membrane-targeted cytosolic domain of STIM1 selectively activates ARC channels, an arachidonate-regulated store-independent Orai channel.
Thompson JL, Shuttleworth TJ. Thompson JL, et al. Channels (Austin). 2012 Sep-Oct;6(5):370-8. doi: 10.4161/chan.21947. Epub 2012 Sep 1. Channels (Austin). 2012. PMID: 22992514 Free PMC article. - STIM1 and STIM2 proteins differently regulate endogenous store-operated channels in HEK293 cells.
Shalygin A, Skopin A, Kalinina V, Zimina O, Glushankova L, Mozhayeva GN, Kaznacheyeva E. Shalygin A, et al. J Biol Chem. 2015 Feb 20;290(8):4717-4727. doi: 10.1074/jbc.M114.601856. Epub 2014 Dec 22. J Biol Chem. 2015. PMID: 25533457 Free PMC article. - Studies of Structure-Function and Subunit Composition of Orai/STIM Channel.
Fahrner M, Schindl R, Romanin C. Fahrner M, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. PMID: 30299645 Free Books & Documents. Review. - Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
Cited by
- ORAI1 Ca2+ Channel as a Therapeutic Target in Pathological Vascular Remodelling.
Shawer H, Norman K, Cheng CW, Foster R, Beech DJ, Bailey MA. Shawer H, et al. Front Cell Dev Biol. 2021 Apr 6;9:653812. doi: 10.3389/fcell.2021.653812. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937254 Free PMC article. Review. - Vascular Smooth Muscle Cell Signaling Mechanisms for Contraction to Angiotensin II and Endothelin-1.
Wynne BM, Chiao CW, Webb RC. Wynne BM, et al. J Am Soc Hypertens. 2009 Mar-Apr;3(2):84-95. doi: 10.1016/j.jash.2008.09.002. J Am Soc Hypertens. 2009. PMID: 20161229 Free PMC article. - TRP channels and Ca2+ signaling.
Minke B. Minke B. Cell Calcium. 2006 Sep;40(3):261-75. doi: 10.1016/j.ceca.2006.05.002. Epub 2006 Jun 27. Cell Calcium. 2006. PMID: 16806461 Free PMC article. Review. - Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1.
Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW Jr. Mercer JC, et al. J Biol Chem. 2006 Aug 25;281(34):24979-90. doi: 10.1074/jbc.M604589200. Epub 2006 Jun 28. J Biol Chem. 2006. PMID: 16807233 Free PMC article. - Regulation of CRAC channel activity by recruitment of silent channels to a high open-probability gating mode.
Prakriya M, Lewis RS. Prakriya M, et al. J Gen Physiol. 2006 Sep;128(3):373-86. doi: 10.1085/jgp.200609588. J Gen Physiol. 2006. PMID: 16940559 Free PMC article.
References
- Berridge M. J., Lipp P., Bootman M. D. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. - PubMed
- Berridge M. J., Bootman M. D., Roderick H. L. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. - PubMed
- Parekh A. B., Penner R. Physiol. Rev. 1997;77:901–930. - PubMed
- Putney J. W., Jr., Broad L. M., Braun F. J., Lievremont J. P., Bird G. S. J. Cell Sci. 2001;114:2223–2229. - PubMed
- Venkatachalam K., van Rossum D. B., Patterson R. L., Ma H. T., Gill D. L. Nat. Cell Biol. 2002;4:E263–E272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous