Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2 - PubMed (original) (raw)
Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2
Hong Liu et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Akt1 is frequently up-regulated in human tumors and has been shown to accelerate cell proliferation and to suppress programmed cell death; consequently, inhibition of the activity of Akt1 has been seen as an attractive target for therapeutic intervention. Paradoxically, hyperactivation of the Akt1 oncogene can also prevent the invasive behavior that underlies progression to metastasis. Here we show that overexpression of activated myr-Akt1 in human breast cancer cells phosphorylates and thereby targets the tumor suppressor tuberous sclerosis complex 2 (TSC2) for degradation, leading to reduced Rho-GTPase activity, decreased actin stress fibers and focal adhesions, and reduced motility and invasion. Overexpression of TSC2 rescues the migration phenotype of myr-Akt1-expressing tumor cells, and high levels of TSC2 in breast cancer patients correlate with increased metastasis and reduced survival. These data indicate that the functional properties of genes designated as oncogenes or tumor suppressor genes depend on the context of the cell type and the tissues studied, and suggest the need for caution in designing therapies targeting the function of individual genes in epithelial tissues.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
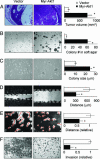
Fig. 1.
Activated Akt1 promotes growth and anchorage independence in T4-2 cells but suppresses motility and invasion. Cells expressing myr-Akt1 showed increased tumor volume in nude mouse xenografts (A), increased colony number in methyl cellulose (B), and increased colony size in soft agar (C). However, myr-Akt1 inhibited cell migration (D) and motility (E), as well as invasion (F). All graphs display averages ± SEM. ∗, P < 0.05.
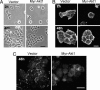
Fig. 2.
Activated Akt1 alters cell-substratum adhesion. Cells expressing myr-Akt1 show reduced cell spreading (A), increased cortical distribution of actin (B), and fewer paxillin-containing focal adhesions (C). (Scale bars: A and C, 10 μm; B, 20 μm.)
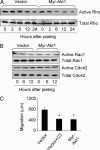
Fig. 3.
Rho GTPases are selectively inhibited by Akt1. Cells were plated for the indicated times, then lysates were analyzed for activity by pull-down with GST-Rhotekin (A) or GST-PAK-CD (B). (C) Inhibition of Rho by pretreatment with recombinant C3 transferase (10 μg/ml) reduced migration of vector control cells in scratch assay to levels of myr-Akt1-expressing cells. The graph displays the average ± SEM. ∗, P < 0.05.
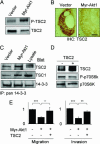
Fig. 4.
TSC2 mediates the effects of Akt1 on motility and invasion. (A) TSC2 shows increased phosphorylation but decreased total levels in cells expressing myr-Akt1. (B) Decreased levels of TSC2 in tumor xenografts from myr-Akt1 cells. (Scale bar: 50 μm.) (C) TSC2 shows increased association with 14-3-3 in cells expressing myr-Akt1. (D) Expression of functional TSC2 in myr-Akt1 cells as shown by reduced phosphorylation of p70S6K. (E) Expression of TSC2 rescues cell invasiveness and motility in myr-Akt1 cells. The graphs display averages ± SEM. ∗, P < 0.05; ∗∗, P < 0.02; ∗∗∗, P < 0.001.
Fig. 5.
Analysis of pathway implication on human breast cancer metastasis. (A) Pathway model. a, Active Akt1 phosphorylates TSC2, stimulating down-modulation of TSC2 levels; b, TSC2 activates Rho; c, Active Rho promotes cytoskeletal rearrangements permissive for cell invasion. (B) Metastasis-free survival of human breast tumors stratified by expression of Akt1 and TSC2. Higher expression of TSC2 together with low expression of Akt1 is predictive of decreased time to metastasis in breast tumors.
Similar articles
- RhoBTB2 (DBC2) functions as tumor suppressor via inhibiting proliferation, preventing colony formation and inducing apoptosis in breast cancer cells.
Mao H, Zhang L, Yang Y, Sun J, Deng B, Feng J, Shao Q, Feng A, Song B, Qu X. Mao H, et al. Gene. 2011 Oct 15;486(1-2):74-80. doi: 10.1016/j.gene.2011.07.018. Epub 2011 Jul 23. Gene. 2011. PMID: 21801820 - Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway.
Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Meng Q, et al. Cell Signal. 2006 Dec;18(12):2262-71. doi: 10.1016/j.cellsig.2006.05.019. Epub 2006 Jun 2. Cell Signal. 2006. PMID: 16839745 - Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton.
Jiang P, Enomoto A, Takahashi M. Jiang P, et al. Cancer Lett. 2009 Nov 1;284(2):122-30. doi: 10.1016/j.canlet.2009.02.034. Epub 2009 Mar 19. Cancer Lett. 2009. PMID: 19303207 Review. - A knotty turnabout?: Akt1 as a metastasis suppressor.
Wyszomierski SL, Yu D. Wyszomierski SL, et al. Cancer Cell. 2005 Dec;8(6):437-9. doi: 10.1016/j.ccr.2005.11.006. Cancer Cell. 2005. PMID: 16338656 Review.
Cited by
- Akt2 regulates expression of the actin-bundling protein palladin.
Chin YR, Toker A. Chin YR, et al. FEBS Lett. 2010 Dec 1;584(23):4769-74. doi: 10.1016/j.febslet.2010.10.056. Epub 2010 Nov 2. FEBS Lett. 2010. PMID: 21050850 Free PMC article. - Inhibition of phosphatidylinositol 3-kinase/Akt signaling suppresses tumor cell proliferation and neuroendocrine marker expression in GI carcinoid tumors.
Pitt SC, Chen H, Kunnimalaiyaan M. Pitt SC, et al. Ann Surg Oncol. 2009 Oct;16(10):2936-42. doi: 10.1245/s10434-009-0591-5. Epub 2009 Jul 9. Ann Surg Oncol. 2009. PMID: 19588205 Free PMC article. - Distinct actions of akt1 on skeletal architecture and function.
Mukherjee A, Larson EA, Klein RF, Rotwein P. Mukherjee A, et al. PLoS One. 2014 Mar 24;9(3):e93040. doi: 10.1371/journal.pone.0093040. eCollection 2014. PLoS One. 2014. PMID: 24663486 Free PMC article. - Assessment of the SRC Inhibition Role in the Efficacy of Breast Cancer Radiotherapy.
Shahrokh S, Mansouri V, Razzaghi M. Shahrokh S, et al. J Lasers Med Sci. 2019 Fall;10(Suppl 1):S18-S22. doi: 10.15171/jlms.2019.S4. Epub 2019 Dec 1. J Lasers Med Sci. 2019. PMID: 32021668 Free PMC article. Review. - Cell migration and invasion assays as tools for drug discovery.
Hulkower KI, Herber RL. Hulkower KI, et al. Pharmaceutics. 2011 Mar 11;3(1):107-24. doi: 10.3390/pharmaceutics3010107. Pharmaceutics. 2011. PMID: 24310428 Free PMC article.
References
- Chambers A. F., Groom A. C., MacDonald I. C. Nat. Rev. Cancer. 2002;2:563–572. - PubMed
- Friedl P., Wolf K. Nat. Rev. Cancer. 2003;3:362–374. - PubMed
- Jaffe A. B., Hall A. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. - PubMed
- Gildea J. J., Harding M. A., Seraj M. J., Gulding K. M., Theodorescu D. Cancer Res. 2002;62:982–985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous