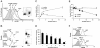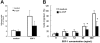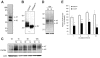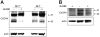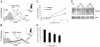G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells - PubMed (original) (raw)
G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells
Hyun Kyung Kim et al. Blood. 2006.
Abstract
CXCR4 receptor expression is required for the retention of granulocyte precursors and mature neutrophils within the bone marrow, and disruption of the SDF-1/CXCR4 axis in the bone marrow results in the mobilization of myeloid lineage cells to the peripheral circulation. We report that G-CSF down-regulates CXCR4 expression in bone marrow-derived murine and human myeloid lineage cells. When exposed to G-CSF, murine Gr1(+) bone marrow myeloid cells display a time-dependent reduction of cell-surface CXCR4 and respond poorly to SDF-1 in attachment and migration assays. Bone marrow-derived cells of nonmyeloid lineage display no change in surface CXCR4 expression upon exposure to G-CSF. Compared with controls, mice treated with G-CSF for mobilization of hematopoietic progenitor cells display reduced levels of CXCR4 selectively in bone marrow Gr1(+) myeloid cells. Since bone marrow myeloid cells express G-CSF receptors and G-CSF rapidly reduces CXCR4 expression in purified Gr1(+) cells populations, these results provide evidence that G-CSF acts directly on myeloid lineage cells to reduce CXCR4 expression. By down-regulating CXCR4 expression in bone marrow myeloid cells and attenuating their responsiveness to SDF-1, G-CSF promotes their mobilization from the bone marrow to the peripheral blood.
Figures
Figure 1.
G-CSF selectively reduces levels of surface CXCR4 in myeloid lineage cells. (A) Flow cytometric analysis of surface CXCR4 expression in bone marrow Gr1+ myeloid cells. Unfractionated bone marrow cell populations were incubated (1 × 106/mL) at 37°C for 6 hours in medium alone or with G-CSF at concentrations of 0.1, 10, and 100 ng/mL. Cells were double stained for Gr1 (APC-labeled) and CXCR4 (FITC-labeled 2B11 antibody) or with APC- and FITC-labeled control antibodies. Gr1+ cells (R1 gate) represented 40.1% of total nucleated cells. (B) Analysis of CXCR4 expression in CD19+ B-lineage (top panel) and CD3e+ T-lineage (bottom panel) cells. Unfractionated bone marrow cell populations were incubated at 37°C for 6 hours with or without G-CSF (100 ng/mL). Cells were double stained for CD19 (APC-labeled) and CXCR4 (FITC-labeled) or CD3e (APC-labeled) and CXCR4 (FITC-labeled) and gated on CD19+ cells (44% of total nucleated cells) or CD3e+ (5.2% of total nucleated cells) cells. (C) Expression of CXCR4 in Gr1+ cells as a function of culture time (0-18 hours) in medium alone or with G-CSF (100 ng/mL). The results are expressed as mean fluorescence intensity (MFI) of CXCR4 detected on Gr1+ cells (mean ± SEM of 3 experiments). (D) Time-dependent reduction of surface CXCR4 in Gr1+ cells induced by G-CSF (100 ng/mL). Unfractionated bone marrow cell populations were incubated at 37°C for 18 hours with or without G-CSF (100 ng/mL). The results are expressed as the mean (± SEM of 5 experiments) percent reduction of surface CXCR4 induced by G-CSF compared with medium alone. *P < .05 medium versus G-CSF. (E) G-CSF reduces surface CXCR4 in precultured bone marrow cells. Unfractionated bone marrow cell populations were preincubated (37°C, 18 hours) in medium alone to achieve high-level surface CXCR4 expression, and further incubated for 18 hours with or without G-CSF (100 ng/mL). The results reflect levels of surface CXCR4 expression (expressed as MFI) in Gr1+ cells cultured with or without G-CSF after the preculture (mean ± SEM of 3 experiments). (F) Unfractionated bone marrow cell populations were incubated (37°C) for 18 hours with or without G-CSF (100 ng/mL), or for 40 minutes with or without SDF-1α (400 ng/mL) to achieve a reduction of surface CXCR4. After washing (PBS containing 50 mM glycine), the cells were incubated for 2 hours at 37°C in medium containing cycloheximide (100 μg/mL). The results reflect surface CXCR4 expression in Gr1+ myeloid cells (representative experiment of 3 performed).
Figure 2.
Effect of G-CSF on bone marrow myeloid cell adhesion and migration to SDF-1. Unfractionated bone marrow cell populations (1 × 106/mL) were preincubated at 37°C for 18 hours with or without G-CSF (100 ng/mL). (A) After washing, the cells were plated onto SDF-1–coated (4 μg/mL) or diluent only–coated (PBS with 0.1% BSA) microtiter wells and incubated at 37°C for 30 minutes. After removal of nonadherent cells, the remaining cells were detached with 5 mM EDTA, counted, and stained for surface Gr1. The results reflect the differential attachment of Gr1+ cells preincubated in medium only or with G-CSF and are expressed as the mean (± SE) percent Gr1+ cells attached of input Gr1+ cells (results from 5 experiments). (B) After preincubation with or without G-CSF and washing, cells were tested for trans-well migration to SDF-1 (2.4-300 ng/mL) or medium only. Cells recovered in the lower well after 2-hour incubation at 37°C were counted and stained for surface Gr1 expression. Results are expressed as the mean (± SE) percent of migrated Gr1+ cells (results reflect the means from 3 experiments). *P < .05 (cells preincubated with medium versus G-CSF).
Figure 3.
Analysis of the relative contribution of G-CSF and soluble mediators induced by G-CSF to the reduction of surface CXCR4 on bone marrow myeloid cells. Unfractionated bone marrow cell populations were preincubated (1 × 106/mL) at 37°C for 18 hours in medium alone or with G-CSF (100 ng/mL). (A) Cell-free supernatants from these cultures were preincubated (1 hour, 37°C) with rabbit neutralizing antibody against human (final concentration, 5 μg/mL) and rat neutralizing antibodies against mouse (final concentration, 3 μg/mL) G-CSF or with PBS only, and then added undiluted (1 mL) to freshly isolated bone marrow cell populations (1 × 106 cells); the mixture was further incubated (6 hours, 37°C). Levels of CXCR4 expression in Gr1+ cells were measured by flow cytometry. The results are expressed as MFI (± SE) of triplicate determinations. *P < .05 (medium versus G-CSF). (B) Detection of G-CSF bioactivity in culture supernatants from bone marrow cell populations incubated with or without neutralizing antibodies against mouse and human G-CSF (as described under “Preparation of murine and human bone marrow cells”). G-CSF bioactivity was measured by 3H thymidine incorporation in murine NFS-60 cells, and the results are expressed as mean (± SE) of triplicate cultures. *P < .05. (C-D) Neutrophil elastase reduces levels of surface CXCR4 in bone marrow myeloid (C) and lymphoid (D) cells. Unfractionated bone marrow cell populations were incubated (1 × 106/mL in DMEM medium with 0.2% BSA for 4 hours at 37°C) with medium alone, leukocyte elastase (enzyme, 40 μg/mL), and with leukocyte elastase plus leukocyte elastase inhibitor III (10 μM). Levels of surface CXCR4 were measured by flow cytometry on Gr1+ cells and lymphoid cells. (E) Dose dependency of leukocyte elastase–induced reduction of surface CXCR4 on Gr1+ cells and lymphoid cells. Unfractionated bone marrow cell populations were incubated with leukocyte elastase (5-100 μg/mL) for 2 hours at 37°C. The results are expressed as percent CXCR4 expression compared with untreated cells (MFI of elastase-treated cells/MFI of untreated cells × 100). (F) Surface CXCR4 expression in the presence of specific enzyme inhibitors. Unfractionated bone marrow cell populations were preincubated (2 hours, 37°C) in medium only (DMEM with 0.2% BSA); or with leukocyte elastase inhibitor III (LEI 10 μM); cathepsin-G (CGI, 10 μM); LEI plus CGI (10 μM each); the CD26/dipeptdyl peptidase inhibitor AB192 (50 μM); and the metalloproteinase inhibitor BB94 (10 μM). Subsequently, cells were incubated with or without G-CSF (100 ng/mL, 4 hours, 37°C). Surface CXCR4 was measured on Gr1+ cells by flow cytometry. The results are expressed as percent MFI compared with cells incubated in medium alone without inhibitors.
Figure 4.
Effects of G-CSF on CXCR4 protein levels in murine bone marrow myeloid cells. (A) Typical representation of CXCR4 protein in cell lysates of freshly isolated Gr1+ cells purified from bone marrow cell populations detected by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with specific antibodies to CXCR4 (2B11 antibody). (B) Effect of the enzyme PNGase F (10 U/40 mg protein) on CXCR4 protein detected by immunoblotting (2B11 antibody). (C) Time-dependent reduction of Gr1+ cell–associated CXCR4 induced by G-CSF. Cells lysates were prepared from purified Gr1+ bone marrow cells either immediately after purification or after culture in medium alone (M) or with G-CSF (G, 100 ng/mL). Samples were immunoblotted with anti-CXCR4 antibodies (2B11), stripped, and reprobed with antibodies to beta-actin (representative experiment of 5 performed). (D) Appearance of CXCR4-related bands after 18-hour incubation of electronically sorted Gr1+ cells (> 95% purity) in medium only (M) or with G-CSF (G, 100 ng/mL). Bottom panel reflects actin-related bands upon reprobing with anti–beta actin antibodies. (E) Levels of CXCR4 protein in Gr1+ cells immediately after purification and after culture (1-18 hours) with medium only or with 100 ng/mL G-CSF measured by band intensities relative to actin. The results are expressed as percent CXCR4 measured in Gr1+ cells immediately after purification (mean ± SD of 3-6 separate experiments; 1-hour time point: mean of 6 experiments; all other time points: mean of 3 experiments). *P < .05; **P < .1 (medium vs G-CSF).
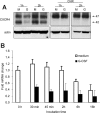
Figure 5.
G-CSF down-regulates CXCR4 mRNA expression in bone marrow myeloid cells. (A) Effects of cycloheximide (CHX) on CXCR4 protein levels in Gr1+ cells detected by immunoblotting with specific antibodies to CXCR4 and actin. Purified bone marrow Gr1+ cells were cultured (1 × 106/mL) for 1 and 2 hours in medium alone (M) or with G-CSF (G, 100 ng/mL), with or without CHX (5 μg/mL). Total cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted (representative experiment of 3 performed). (B) Levels of CXCR4 mRNA in purified bone marrow Gr1+ cells immediately after purification and after culture with G-CSF (100 ng/mL, 30 minutes to 16 hours) measured by real-time RT-PCR analysis. The results are expressed as mean fold (± SE) mRNA change relative to mRNA levels detected before culture (results from 3 independent experiments each tested in triplicate).
Figure 6.
G-CSF–induced hematopoietic cell mobilization is associated with a reduction of CXCR4 in myeloid cells. Bone marrow Gr1+ cells were purified from the bone marrow of groups of mice (C57/BL6) either untreated or treated for 5 days with G-CSF (5 μg/mouse, daily intraperitoneally for 5 days). (A) Gr1+ cells and Gr1– cells were obtained from groups of mice treated for 5 days with G-CSF or buffer only. CXCR4 content in Gr1+ cells and Gr1– cell lysates evaluated by immunoblotting with specific CXCR4 antibodies, and reprobing with anti–beta actin antibodies. (B) Western blot analysis of CXCR4 and actin content in cell lysates from 2 groups of mice treated with G-CSF and a control group of untreated mice.
Figure 7.
Effects of G-CSF on CXCR4 expression in human bone marrow myeloid cells. (A-B) Unfractionated bone marrow cell populations were incubated (1 × 106/mL) at 37°C for 18 hours in medium alone or with G-CSF (1 and 100 ng/mL). Cells were double stained for CD33 (PE-labeled) and CXCR4 (APC-labeled 12G5 antibody) or with PE- and APC-labeled control antibodies (isotype). (A) Surface CXCR4 expression in human bone marrow CD33+ myeloid cells cultured for 18 hours in medium alone (none) or with G-CSF (1 and 100 ng/mL). (B) Surface CXCR4 expression in human bone marrow CD33– cell populations cultured for 18 hours in medium alone (none) or with G-CSF (1 and 100 ng/mL). (C) Expression of surface CXCR4 in human CD33+ bone marrow cells as a function of culture time (0-18 hours) in medium alone or with G-CSF (100 ng/mL). The results are expressed as mean fluorescence intensity (MFI) of CXCR4 detected on CD33+ cells (mean ± SEM of 3 experiments). (D) Time-dependent reduction of surface CXCR4 in CD33+ cells induced by G-CSF (100 ng/mL). Unfractionated bone marrow cell populations were incubated at 37°C for 18 hours with or without G-CSF (100 ng/mL). The results are expressed as the mean (± SEM of 3 experiments) percent reduction of surface CXCR4 induced by G-CSF compared with medium alone. *P < .05 medium versus G-CSF. (E) CXCR4 detected in cell lysates of purified bone marrow CD33+ cells (> 85% purity) immediately after separation (0h), and after 1.5-hour and 18-hour incubation in medium only (M) or with G-CSF (G, 100 ng/mL). Bottom panel reflects the results upon membrane reprobing with anti–beta actin antibodies.
Similar articles
- Transcription factor Gfi-1 induced by G-CSF is a negative regulator of CXCR4 in myeloid cells.
De La Luz Sierra M, Gasperini P, McCormick PJ, Zhu J, Tosato G. De La Luz Sierra M, et al. Blood. 2007 Oct 1;110(7):2276-85. doi: 10.1182/blood-2007-03-081448. Epub 2007 Jun 27. Blood. 2007. PMID: 17596540 Free PMC article. - [The role of stromal cell-derived factor and its receptor-CXCR4 in G-CSF-induced hematopoietic stem cell mobilization].
Jin FY, Qiu LG, Li QC, Meng HX, Wang YF, Yu Z, Li Q, Han JL. Jin FY, et al. Zhonghua Xue Ye Xue Za Zhi. 2007 Feb;28(2):98-102. Zhonghua Xue Ye Xue Za Zhi. 2007. PMID: 17650669 Chinese. - [The role of the SDF-1-CXCR4 axis in hematopoiesis and the mobilization of hematopoietic stem cells to peripheral blood].
Gieryng A, Bogunia-Kubik K. Gieryng A, et al. Postepy Hig Med Dosw (Online). 2007 Jun 13;61:369-83. Postepy Hig Med Dosw (Online). 2007. PMID: 17572657 Review. Polish. - Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells.
Lapidot T, Petit I. Lapidot T, et al. Exp Hematol. 2002 Sep;30(9):973-81. doi: 10.1016/s0301-472x(02)00883-4. Exp Hematol. 2002. PMID: 12225788 Review.
Cited by
- Granulocyte colony-stimulating factor inhibits CXCR4/SDF-1α signaling and overcomes stromal-mediated drug resistance in the HL-60 cell line.
Sheng X, Zhong H, Wan H, Zhong J, Chen F. Sheng X, et al. Exp Ther Med. 2016 Jul;12(1):396-404. doi: 10.3892/etm.2016.3268. Epub 2016 Apr 20. Exp Ther Med. 2016. PMID: 27347068 Free PMC article. - Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1-, IL-17-, and G-CSF-dependent manner.
Capitano ML, Nemeth MJ, Mace TA, Salisbury-Ruf C, Segal BH, McCarthy PL, Repasky EA. Capitano ML, et al. Blood. 2012 Sep 27;120(13):2600-9. doi: 10.1182/blood-2012-02-409805. Epub 2012 Jul 17. Blood. 2012. PMID: 22806894 Free PMC article. - CXCL8 and its cognate receptors CXCR1/CXCR2 in primary myelofibrosis.
Vermeersch G, Proost P, Struyf S, Gouwy M, Devos T. Vermeersch G, et al. Haematologica. 2024 Jul 1;109(7):2060-2072. doi: 10.3324/haematol.2023.284921. Haematologica. 2024. PMID: 38426279 Free PMC article. Review. - Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells.
Jalili A, Shirvaikar N, Marquez-Curtis L, Qiu Y, Korol C, Lee H, Turner AR, Ratajczak MZ, Janowska-Wieczorek A. Jalili A, et al. Exp Hematol. 2010 Apr;38(4):321-32. doi: 10.1016/j.exphem.2010.02.002. Epub 2010 Feb 12. Exp Hematol. 2010. PMID: 20153802 Free PMC article. - Neutrophil chemoattractant receptors in health and disease: double-edged swords.
Metzemaekers M, Gouwy M, Proost P. Metzemaekers M, et al. Cell Mol Immunol. 2020 May;17(5):433-450. doi: 10.1038/s41423-020-0412-0. Epub 2020 Apr 1. Cell Mol Immunol. 2020. PMID: 32238918 Free PMC article. Review.
References
- Link DC. Neutrophil homeostasis: a new role for stromal cell-derived factor-1. Immunol Res. 2005;32: 169-178. - PubMed
- Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10: 463-471. - PubMed
- Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR Jr. WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000;91: 368-376. - PubMed
- Gulino AV, Moratto D, Sozzani S, et al. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004;104: 444-452. - PubMed
- Balabanian K, Lagane B, Pablos JL, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105: 2449-2457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources