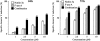Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia - PubMed (original) (raw)
Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia
Kensuke Kojima et al. Blood. 2006.
Abstract
Although TP53 mutations are rare in B-cell chronic lymphocytic leukemia (CLL), Mdm2 overexpression has been reported as an alternative cause of p53 dysfunction. We investigated the potential therapeutic use of nongenotoxic p53 activation by a small-molecule antagonist of Mdm2, Nutlin-3a, in CLL. Nutlin-3a induced significant apoptosis in 30 (91%) of 33 samples from previously untreated patients with CLL; all resistant samples had TP53 mutations. Low levels of Atm (ataxia telangiectasia mutated) or high levels of Mdm2 (murine double minute 2) did not prevent Nutlin-3a from inducing apoptosis. Nutlin-3a used transcription-dependent and transcription-independent pathways to induce p53-mediated apoptosis. Predominant activation of the transcription-independent pathway induced more pronounced apoptosis than that of the transcription-dependent pathway, suggesting that activation of the transcription-independent pathway is sufficient to initiate p53-mediated apoptosis in CLL. Combination treatment of Nutlin-3a and fludarabine synergistically increased p53 levels, and induced conformational change of Bax and apoptosis in wild-type p53 cells but not in cells with mutant p53. The synergistic apoptotic effect was maintained in samples with low Atm that were fludarabine resistant. Results suggest that the nongenotoxic activation of p53 by targeting the Mdm2-p53 interaction provides a novel therapeutic strategy for CLL.
Figures
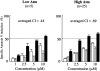
Figure 1.
Treatment of primary CLL samples with Nutlin-3a or F-ara-A causes dose- and time-dependent apoptosis, and their combination shows synergistic effects. Cells from 30 Nutlin-sensitive samples were incubated with the indicated concentrations of Nutlin-3a or F-ara-A, and the annexin V–positive fractions were measured by flow cytometry at 24 and 72 hours. Results are expressed as mean ± SEM.
Figure 2.
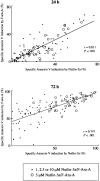
Positive correlation of Nutlin-3a– and F-ara-A–induced apoptosis in CLL patient samples. The extent of apoptosis induced by 1, 2.5, 5, or 10 μM Nutlin-3a was correlated with that induced by the same molar concentration of F-ara-A in 30 Nutlin-sensitive samples.
Figure 3.
Induction of p53-related proteins by F-ara-A and Nutlin-3a in a CLL sample with wild-type p53. The sample contained more than 95% CD5+CD19+ cells with normal Atm levels. Cells were treated with 5 μM F-ara-A (F) and 5 μM Nutlin-3a (3a) either as individual agents or in combination, for the indicated times. F-ara-A induced p53 phosphorylation on Ser, followed by p53 accumulation and Puma induction. Nutlin-3a induced immediate accumulation of p53 that is mostly free of phosphorylation on Ser, followed by Mdm2 and Puma induction. The degree of Puma induction was further enhanced in cells treated with F-ara-A and Nutlin-3a. β-Actin is used to confirm equal loading of proteins. Lysate from cells before treatment served as control (C).
Figure 4.
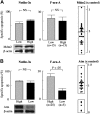
Synergistic induction of p53 signaling by F-ara-A and Nutlin-3a in CLL cells with wild-type p53. (A) Synergistic induction of p53 by F-ara-A and Nutlin-3a in CLL cells with wild-type p53. Samples from 4 wild-type p53 patients (nos. 4, 10, 11, and 30; no. 10 showed reduced Atm expression) and from a mutant p53 patient (no. 28) were treated for 24 hours with 5 μM F-ara-A (F) and 5 μM Nutlin-3a (3a) either as individual agents or in combination. DMSO-treated cells served as control (C). Shaded histograms represent isotype controls. Results show that F-ara-A and Nutlin-3a synergistically induce p53 in cases with wild-type p53, irrespective of Atm status. p53 expression levels were expressed as mean fluorescence intensity (MFI) ratio calculated by the following formula: MFI ratio = (MFI for anti-p53 antibody)/(MFI for isotypic control). WT indicates wild-type; MUT, mutant; N, normal; R, reduced; and ND, not done. (B) A synergistic activation of Bax by F-ara-A and Nutlin-3a in CLL cells with wild-type p53. Cells from patient no. 27 (wild-type p53) and from patient no. 28 (mutant p53) were treated for 24 hours with 5 μM F-ara-A (F) and 5 μM Nutlin-3a (3a) either as individual agents or in combination. Bax conformational change was determined by staining with the active conformation-specific anti-Bax antibody YTH-6A7 or a corresponding isotype control (shaded histogram). Z-VAD-FMK (200 μM) was used to inhibit caspase activation–mediated conformational change of Bax, and cells treated with Z-VAD-FMK alone served as control (C). Results show a synergistic activation of Bax by F-ara-A and Nutlin-3a in wild-type p53 but not in mutant p53 cells.
Figure 5.
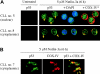
p53 relocation after Nutlin-3a treatment. (A) Representative p53 localization patterns (arrows) in primary CLL cells from patient no. 5 (nuclear accumulation) and no. 8 (cytoplasmic accumulation), which were treated with 5 μM Nutlin-3a for 6 hours. Cells were stained for p53 (green) and mitochondrial marker protein cytochrome c oxidase IV (red) and visualized by confocal microscopy. Nuclei were counterstained with DAPI (blue). (B) Preferential translocation of cytoplasmic p53 to mitochondria in CLL cells. Localization of p53 to mitochondria is indicated by the yellow-orange color in the merged image (arrowheads).
Figure 6.
Nutlin-3a efficiently induces p53-mediated apoptosis in CLL cells independent of Mdm2 or Atm expression levels. (A) High levels of Mdm2 were not associated with resistance to Nutlin-3a– or F-ara-A–induced apoptosis in CLL. Mdm2 protein expression levels relative to an internal control, β-actin, were determined in each sample and compared with normal bone marrow cells. (B) Low Atm levels did not prevent Nutlin-3a from inducing apoptosis in CLL. Atm protein expression levels relative to an internal control, β-actin, were determined in each sample, and compared with normal peripheral-blood lymphocytes. Although low Atm expression was associated with fludarabine resistance in 5 cases, these samples remained sensitive to Nutlin-induced apoptosis. Error bars indicate SEM.
Figure 7.
Synergism between Nutlin-3a and F-ara-A is maintained in low Atm cases. Results of annexin V–binding assay in 30 Nutlin-sensitive samples (Figure 1) were separately analyzed with regard to Atm expression levels. Cells were incubated with the indicated concentrations of Nutlin-3a or F-ara-A, and the annexin V–positive fractions were measured by flow cytometry at 24 hours. Results are expressed as mean ± SEM. □ indicates Nutlin-3a;  , F-ara-A; and ▪, combination.
, F-ara-A; and ▪, combination.
Similar articles
- Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1.
Ambrosini G, Sambol EB, Carvajal D, Vassilev LT, Singer S, Schwartz GK. Ambrosini G, et al. Oncogene. 2007 May 24;26(24):3473-81. doi: 10.1038/sj.onc.1210136. Epub 2006 Dec 4. Oncogene. 2007. PMID: 17146434 - Functional integrity of the p53-mediated apoptotic pathway induced by the nongenotoxic agent nutlin-3 in B-cell chronic lymphocytic leukemia (B-CLL).
Secchiero P, Barbarotto E, Tiribelli M, Zerbinati C, di Iasio MG, Gonelli A, Cavazzini F, Campioni D, Fanin R, Cuneo A, Zauli G. Secchiero P, et al. Blood. 2006 May 15;107(10):4122-9. doi: 10.1182/blood-2005-11-4465. Epub 2006 Jan 26. Blood. 2006. PMID: 16439677 - Decreased sensitivity of 17p-deleted chronic lymphocytic leukemia cells to a small molecule BCL-2 antagonist ABT-737.
Kojima K, Duvvuri S, Ruvolo V, Samaniego F, Younes A, Andreeff M. Kojima K, et al. Cancer. 2012 Feb 15;118(4):1023-31. doi: 10.1002/cncr.26360. Epub 2011 Jul 14. Cancer. 2012. PMID: 21761401 Free PMC article. - Resistance mechanisms to inhibitors of p53-MDM2 interactions in cancer therapy: can we overcome them?
Haronikova L, Bonczek O, Zatloukalova P, Kokas-Zavadil F, Kucerikova M, Coates PJ, Fahraeus R, Vojtesek B. Haronikova L, et al. Cell Mol Biol Lett. 2021 Dec 15;26(1):53. doi: 10.1186/s11658-021-00293-6. Cell Mol Biol Lett. 2021. PMID: 34911439 Free PMC article. Review. - Targeting TP53 disruption in chronic lymphocytic leukemia: Current strategies and future directions.
Molica S, Tam C, Allsup D, Polliack A. Molica S, et al. Hematol Oncol. 2024 Jan;42(1):e3238. doi: 10.1002/hon.3238. Epub 2023 Nov 8. Hematol Oncol. 2024. PMID: 37937506 Review.
Cited by
- Bax, Bcl2, and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line.
Coffin AB, Rubel EW, Raible DW. Coffin AB, et al. J Assoc Res Otolaryngol. 2013 Oct;14(5):645-59. doi: 10.1007/s10162-013-0404-1. Epub 2013 Jul 3. J Assoc Res Otolaryngol. 2013. PMID: 23821348 Free PMC article. - High-throughput proteomic profiling reveals mechanisms of action of AMG925, a dual FLT3-CDK4/6 kinase inhibitor targeting AML and AML stem/progenitor cells.
Zeng Z, Ly C, Daver N, Cortes J, Kantarjian HM, Andreeff M, Konopleva M. Zeng Z, et al. Ann Hematol. 2021 Jun;100(6):1485-1496. doi: 10.1007/s00277-021-04493-0. Epub 2021 Mar 31. Ann Hematol. 2021. PMID: 33787984 - The pathophysiological significance of PPM1D and therapeutic targeting of PPM1D-mediated signaling by GSK2830371 in mantle cell lymphoma.
Kojima K, Maeda A, Yoshimura M, Nishida Y, Kimura S. Kojima K, et al. Oncotarget. 2016 Oct 25;7(43):69625-69637. doi: 10.18632/oncotarget.11904. Oncotarget. 2016. PMID: 27626308 Free PMC article. - Phase 1 dose escalation study of the MDM2 inhibitor milademetan as monotherapy and in combination with azacitidine in patients with myeloid malignancies.
DiNardo CD, Olin R, Wang ES, Skikne B, Rosenthal J, Kumar P, Sumi H, Hizukuri Y, Hong Y, Patel P, Seki T, Duan T, Lesegretain A, Andreeff M. DiNardo CD, et al. Cancer Med. 2024 Jul;13(14):e70028. doi: 10.1002/cam4.70028. Cancer Med. 2024. PMID: 39030997 Free PMC article. Clinical Trial. - Pharmacological activation of wild-type p53 in the therapy of leukemia.
Kojima K, Ishizawa J, Andreeff M. Kojima K, et al. Exp Hematol. 2016 Sep;44(9):791-798. doi: 10.1016/j.exphem.2016.05.014. Epub 2016 Jun 18. Exp Hematol. 2016. PMID: 27327543 Free PMC article. Review.
References
- Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103: 4389-4395. - PubMed
- Johnson S, Smith AG, Loffler H, et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia: The French Cooperative Group on CLL. Lancet. 1996;347: 1432-1438. - PubMed
- Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343: 1750-1757. - PubMed
- Leporrier M, Chevret S, Cazin B, et al. Randomized comparison of fludarabine, CAP, and ChOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001;98: 2319-2325. - PubMed
- Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105: 49-53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous