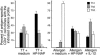The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses - PubMed (original) (raw)
. 2006 Apr;116(4):1092-101.
doi: 10.1172/JCI27177. Epub 2006 Mar 16.
Affiliations
- PMID: 16543949
- PMCID: PMC1401483
- DOI: 10.1172/JCI27177
The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses
Amedeo Amedei et al. J Clin Invest. 2006 Apr.
Abstract
The Helicobacter pylori neutrophil-activating protein (HP-NAP) is a virulence factor of H. pylori that stimulates in neutrophils high production of oxygen radicals and adhesion to endothelial cells. We report here that HP-NAP is a TLR2 agonist able to induce the expression of IL-12 and IL-23 by neutrophils and monocytes. Addition in culture of HP-NAP, as an immune modulator, to antigen-induced T cell lines resulted in a remarkable increase in the number of IFN-gamma-producing T cells and decrease of IL-4-secreting cells, thus shifting the cytokine profile of antigen-activated human T cells from Th2 to a Th1 cytotoxic phenotype. We also found that in vivo HP-NAP elicited an antigen-specific Th1-polarized T cell response in the gastric mucosa of H. pylori-infected patients. These data indicate HP-NAP as an important factor of H. pylori able to elicit cells of the innate immune system to produce IL-12 and IL-23, and they suggest it as a new tool for promoting Th1 immune responses.
Figures
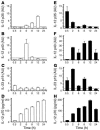
Figure 1. Kinetics of cytokine mRNA levels and IL-12p70 production in neutrophils and monocytes stimulated with HP-NAP.
Cytokine mRNAs in neutrophils (A–C) and monocytes (E–G) were determined by quantitative real-time PCR. The experiment shown is 1 representative of 7 experiments conducted with different cell preparations. The dotted lines represent the level of cytokine mRNA produced by mock cells. IL-12p70 protein levels were measured in the culture supernatants of the same neutrophils (D) and monocytes (H) harvested for messenger evaluation. Levels were assessed by a specific ELISA method. IL-12p70 protein levels at time 0 were under the lower limit of sensitivity of the assay (7.8 pg/ml). The kinetics of production were comparable among different experiments, whereas the amounts varied among different donors.
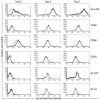
Figure 2. Flow cytometric analysis of HP-NAP–stimulated monocytes and DCs.
Solid and dotted lines correspond to HP-NAP–treated monocytes and to isotype controls, respectively. Results of 2 representative of 4 consecutive experiments are reported.
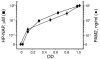
Figure 3. Activation of NF-κB in HEK 293 cells transfected with plasmid encoding human TLR2.
Parallel culture samples of hTLR2-transfected HEK 293 cells were stimulated with graded concentrations of HP-NAP (from 0.03 to 1.0 μM) (squares), or with graded concentrations of the specific hTLR2-positive control ligand PAM2 (from 0.1 to 10 ng/ml) (diamonds). A recombinant HEK 293 cell line for the reporter gene only was used as a negative control (data not shown). The NF-κB activation in each sample was quantified as OD values after 24 hours of stimulation. Results of a representative experiment are reported.
Figure 4. Conditioning with HP-NAP promotes IFN-γ production.
Addition in culture of HP-NAP together with antigen increases IFN-γ–producing T cells and reduces IL-4–secreting cells. TT-induced T cell lines were generated from PBMCs of 5 healthy donors in the presence of medium or HP-NAP. T cell blasts of each line were then stimulated with TT in the presence of irradiated autologous APCs, and IFN-γ– or IL-4–producing T cells were assessed by specific ELISPOT assays. Results represent mean numbers (± SD) of SFCs per million cultured cells counted using an automated ELISPOT reader.
Figure 5. Conditioning with HP-NAP promotes the Th1 polarization of antigen-specific T cells.
TT- or allergen-induced T cell lines (left and right panels, respectively) were generated in the presence of medium alone, HP-NAP, or IL-12. T cell blasts of each line were then cloned, and antigen-specific T cell clones were stimulated for 48Πhours with medium or the appropriate antigen in the presence of irradiated autologous APCs. Culture supernatants were then collected and assayed for their IFN-γ and IL-4 content. Clones able to produce IFN-γ, but not IL-4, were categorized as Th1; clones producing IL-4, but not IFN-γ, were coded as Th2, whereas clones producing both IFN-γ and IL-4 were categorized as Th0. Results represent mean percentage proportions (± SD) of clones with the indicated cytokine profile, obtained from series of 3 T cell lines for each condition.
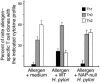
Figure 6. WT, but not HP-NAP–null mutant, H. pylori promotes the Th1 shift of allergen-specific T cells.
Allergen-induced T cell lines were generated in the presence of medium alone, WT H. pylori, or HP-NAP–null H. pylori mutant (5 × 105 CFUs/ml). T cell blasts of each line were then cloned, and allergen-specific T cell clones were stimulated for 48Πhours with medium or allergen in the presence of irradiated autologous APCs. Culture supernatants were then collected and assayed for their IFN-γ and IL-4 content. Results represent mean percentage proportions (± SD) of clones with the indicated cytokine profile, obtained in T cell lines from 3 donors.
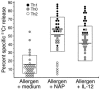
Figure 7. Cytotoxic activity of Th clones derived from allergen-induced T cell lines conditioned with medium alone, HP-NAP, or IL-12.
Results represent the percentage specific 51Cr release induced by single clones in PHA-treated murine 51Cr-labeled P815 mastocytoma cells at an effector-to-target ratio of 5:1. Horizontal bars and boxes indicate mean values ± SD, respectively.
Similar articles
- The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent.
D'Elios MM, Amedei A, Cappon A, Del Prete G, de Bernard M. D'Elios MM, et al. FEMS Immunol Med Microbiol. 2007 Jul;50(2):157-64. doi: 10.1111/j.1574-695X.2007.00258.x. Epub 2007 May 22. FEMS Immunol Med Microbiol. 2007. PMID: 17521355 Review. - VacA and HP-NAP, Ying and Yang of Helicobacter pylori-associated gastric inflammation.
D'Elios MM, Montecucco C, de Bernard M. D'Elios MM, et al. Clin Chim Acta. 2007 May;381(1):32-8. doi: 10.1016/j.cca.2007.02.026. Epub 2007 Feb 21. Clin Chim Acta. 2007. PMID: 17368441 Review. - Vector-encoded Helicobacter pylori neutrophil-activating protein promotes maturation of dendritic cells with Th1 polarization and improved migration.
Ramachandran M, Jin C, Yu D, Eriksson F, Essand M. Ramachandran M, et al. J Immunol. 2014 Sep 1;193(5):2287-96. doi: 10.4049/jimmunol.1400339. Epub 2014 Jul 21. J Immunol. 2014. PMID: 25049358
Cited by
- Immune Biology and Persistence of Helicobacter pylori in Gastric Diseases.
Fuchs S, Gong R, Gerhard M, Mejías-Luque R. Fuchs S, et al. Curr Top Microbiol Immunol. 2023;444:83-115. doi: 10.1007/978-3-031-47331-9_4. Curr Top Microbiol Immunol. 2023. PMID: 38231216 - An infection-enhanced oncolytic adenovirus secreting H. pylori neutrophil-activating protein with therapeutic effects on neuroendocrine tumors.
Ramachandran M, Yu D, Wanders A, Essand M, Eriksson F. Ramachandran M, et al. Mol Ther. 2013 Nov;21(11):2008-18. doi: 10.1038/mt.2013.153. Epub 2013 Jul 2. Mol Ther. 2013. PMID: 23817216 Free PMC article. - Expression of immunomodulatory neutrophil-activating protein of Helicobacter pylori enhances the antitumor activity of oncolytic measles virus.
Iankov ID, Allen C, Federspiel MJ, Myers RM, Peng KW, Ingle JN, Russell SJ, Galanis E. Iankov ID, et al. Mol Ther. 2012 Jun;20(6):1139-47. doi: 10.1038/mt.2012.4. Epub 2012 Feb 14. Mol Ther. 2012. PMID: 22334023 Free PMC article. - HP-NAP of Helicobacter pylori: The Power of the Immunomodulation.
Codolo G, Coletta S, D'Elios MM, de Bernard M. Codolo G, et al. Front Immunol. 2022 Jun 29;13:944139. doi: 10.3389/fimmu.2022.944139. eCollection 2022. Front Immunol. 2022. PMID: 35844568 Free PMC article. Review. - Impairment of ghrelin synthesis in Helicobacter pylori-colonized stomach: new clues for the pathogenesis of H. pylori-related gastric inflammation.
Paoluzi OA, Blanco del VG, Caruso R, Monteleone I, Monteleone G, Pallone F. Paoluzi OA, et al. World J Gastroenterol. 2014 Jan 21;20(3):639-46. doi: 10.3748/wjg.v20.i3.639. World J Gastroenterol. 2014. PMID: 24574737 Free PMC article. Review.
References
- Marshall B.J., Warren J.R. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1984;1:1311–1315. - PubMed
- Goodwin C.S. Helicobacter pylori gastritis, peptic ulcer and gastric cancer: clinical and molecular aspects. . Clin. Infect. Dis. 1997;25:1017–1019. - PubMed
- Parsonnet J., et al. Helicobacter pylori infection and gastric lymphoma. . N. Engl. J. Med. 1994;330:1267–1271. - PubMed
- Dixon M.F., Genta R.M., Yardley J.H., Correa P. Classification and grading of gastritis. . Am. J. Surg. Pathol. 1996;20:1161–1181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous