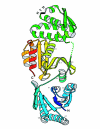A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action - PubMed (original) (raw)
A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action
Kira S Makarova et al. Biol Direct. 2006.
Abstract
Background: All archaeal and many bacterial genomes contain Clustered Regularly Interspaced Short Palindrome Repeats (CRISPR) and variable arrays of the CRISPR-associated (cas) genes that have been previously implicated in a novel form of DNA repair on the basis of comparative analysis of their protein product sequences. However, the proximity of CRISPR and cas genes strongly suggests that they have related functions which is hard to reconcile with the repair hypothesis.
Results: The protein sequences of the numerous cas gene products were classified into approximately 25 distinct protein families; several new functional and structural predictions are described. Comparative-genomic analysis of CRISPR and cas genes leads to the hypothesis that the CRISPR-Cas system (CASS) is a mechanism of defense against invading phages and plasmids that functions analogously to the eukaryotic RNA interference (RNAi) systems. Specific functional analogies are drawn between several components of CASS and proteins involved in eukaryotic RNAi, including the double-stranded RNA-specific helicase-nuclease (dicer), the endonuclease cleaving target mRNAs (slicer), and the RNA-dependent RNA polymerase. However, none of the CASS components is orthologous to its apparent eukaryotic functional counterpart. It is proposed that unique inserts of CRISPR, some of which are homologous to fragments of bacteriophage and plasmid genes, function as prokaryotic siRNAs (psiRNA), by base-pairing with the target mRNAs and promoting their degradation or translation shutdown. Specific hypothetical schemes are developed for the functioning of the predicted prokaryotic siRNA system and for the formation of new CRISPR units with unique inserts encoding psiRNA conferring immunity to the respective newly encountered phages or plasmids. The unique inserts in CRISPR show virtually no similarity even between closely related bacterial strains which suggests their rapid turnover, on evolutionary scale. Corollaries of this finding are that, even among closely related prokaryotes, the most commonly encountered phages and plasmids are different and/or that the dominant phages and plasmids turn over rapidly.
Conclusion: We proposed previously that Cas proteins comprise a novel DNA repair system. The association of the cas genes with CRISPR and, especially, the presence, in CRISPR units, of unique inserts homologous to phage and plasmid genes make us abandon this hypothesis. It appears most likely that CASS is a prokaryotic system of defense against phages and plasmids that functions via the RNAi mechanism. The functioning of this system seems to involve integration of fragments of foreign genes into archaeal and bacterial chromosomes yielding heritable immunity to the respective agents. However, it appears that this inheritance is extremely unstable on the evolutionary scale such that the repertoires of unique psiRNAs are completely replaced even in closely related prokaryotes, presumably, in response to rapidly changing repertoires of dominant phages and plasmids.
Figures
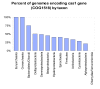
Figure 1
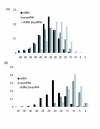
Distribution of COG1518 genes and, by implication, CASS among prokaryotic lineages.
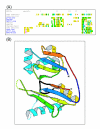
Figure 2
Phylogenies of the key cas genes and organization of cas operons. (a) Phylogenetic tree for COG1518 proteins (b) Phylogenetic tree for COG1203 proteins (predicted helicase) from the CASS versions lacking COG1518 (c) Phylogenetic tree for the predicted CASS polymerase (COG1353). Prokaryotic lineages are color-coded: orange, archaea; blue, Proteobacteria; green, low-GC Gram-positive bacteria; black, other bacteria. In the operon organizations cartoons, orthologous genes are color-coded and denoted by either the predicted function or the COG number. Exclamation points denote previously undetected RAMPs. The names of species that have a reverse transcriptase gene within one of the cas operons are underlined in red. In the left panel, the distinct versions of CASS are numbered, and in the right panel, these numbers are given at tree leaves to indicate the helicase cassette(s) that co-occurs with the given polymerase cassette.
Figure 3
The RAMPs. (A) The conserved motifs of the RAMP superfamily and individual RAMP families. h designates a hydrophobic residue, p designates a polar residues, t designates a residue with high turn-forming propensity, and + designates a positively charged residue. (B) A ribbon model of the structure of a RAMP protein from Thermus thermophilus (PDB entry 1wj9). Two ferredoxin-like domains are rainbow-colored from N- to C-terminus such that the corresponding strands in the two each domain receive the same color. The G-rich conserved loop in the C-terminal domain is colored black, structurally disordered regions are shown by dots, α-helices and β-strands are numbered consecutively throughout the sequence from α1 to α4 and from β1 to β8.
Figure 4
A ribbon model for the structure of a COG1517 protein, Vc1899 from Vibrio cholerae (PDB entry 1xmx). The structure is rainbow-colored from N- to C-terminus such that each of the three domains is assigned a visually distinct region of the color spectrum: blue, the modified Rossmann-like fold; green, the winged helix-turn helix (HTH) domain; yellow-orange, the endonuclease-like domain. The T-turn in the HTH is colored black, a structurally disordered region is shown by dots, α-helices and β-strands are numbered consecutively throughout the sequence from α1 to α14 and from β1 to β16.
Figure 5
The current hypothetical model for CASS functioning and CRISPR formation. (A) The basic model of CASS functioning (B) The variant of CASS functioning involving the CASS polymerase (C) Formation of new CRISPR with unique inserts.
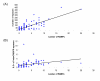
Figure 6
CRISPR and RAMPs. (A) Correlation between the number of encoded RAMPs and the number of CRISPR units in prokaryotic genomes (B) Correlation between the number of encoded RAMPs and the variance of unique insert lengths of CRISPR-related spacers in prokaryotic genomes.
Figure 7
Folding free energy distributions for the putative psiRNA precursors. (a) GC-rich psiRNA precursors compared to the corresponding shuffled sequences and miRNAs (b) AT-rich psiRNA precursors compared to the corresponding shuffled sequences and mRNAs. The X-axis: folding energy.
Figure 8
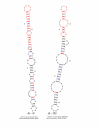
Two predicted structures of putative psiRNA precursors. The unique inserts are shown in red, and the CRISPR sequence is shown in boldface.
Similar articles
- Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements.
Makarova KS, Wolf YI, van der Oost J, Koonin EV. Makarova KS, et al. Biol Direct. 2009 Aug 25;4:29. doi: 10.1186/1745-6150-4-29. Biol Direct. 2009. PMID: 19706170 Free PMC article. - Impact of Different Target Sequences on Type III CRISPR-Cas Immunity.
Maniv I, Jiang W, Bikard D, Marraffini LA. Maniv I, et al. J Bacteriol. 2016 Jan 11;198(6):941-50. doi: 10.1128/JB.00897-15. J Bacteriol. 2016. PMID: 26755632 Free PMC article. - Assessment of the evolutionary origin and possibility of CRISPR-Cas (CASS) mediated RNA interference pathway in Vibrio cholerae O395.
Chakraborty S, Waise TM, Hassan F, Kabir Y, Smith MA, Arif M. Chakraborty S, et al. In Silico Biol. 2009;9(4):245-54. In Silico Biol. 2009. PMID: 20109154 - Role of CRISPR/cas system in the development of bacteriophage resistance.
Szczepankowska A. Szczepankowska A. Adv Virus Res. 2012;82:289-338. doi: 10.1016/B978-0-12-394621-8.00011-X. Adv Virus Res. 2012. PMID: 22420856 Review. - Clustered regularly interspaced short palindromic repeats (CRISPRs): the hallmark of an ingenious antiviral defense mechanism in prokaryotes.
Al-Attar S, Westra ER, van der Oost J, Brouns SJ. Al-Attar S, et al. Biol Chem. 2011 Apr;392(4):277-89. doi: 10.1515/BC.2011.042. Epub 2011 Feb 7. Biol Chem. 2011. PMID: 21294681 Review.
Cited by
- Polyclonality of concurrent natural populations of Alteromonas macleodii.
Gonzaga A, Martin-Cuadrado AB, López-Pérez M, Megumi Mizuno C, García-Heredia I, Kimes NE, Lopez-García P, Moreira D, Ussery D, Zaballos M, Ghai R, Rodriguez-Valera F. Gonzaga A, et al. Genome Biol Evol. 2012;4(12):1360-74. doi: 10.1093/gbe/evs112. Genome Biol Evol. 2012. PMID: 23212172 Free PMC article. - Genomic Insights into Drug Resistance and Virulence Platforms, CRISPR-Cas Systems and Phylogeny of Commensal E. coli from Wildlife.
Alonso CA, de Toro M, de la Cruz F, Torres C. Alonso CA, et al. Microorganisms. 2021 May 5;9(5):999. doi: 10.3390/microorganisms9050999. Microorganisms. 2021. PMID: 34063152 Free PMC article. - CRISPR-Based Diagnosis of Infectious and Noninfectious Diseases.
Jolany Vangah S, Katalani C, Booneh HA, Hajizade A, Sijercic A, Ahmadian G. Jolany Vangah S, et al. Biol Proced Online. 2020 Sep 14;22:22. doi: 10.1186/s12575-020-00135-3. eCollection 2020. Biol Proced Online. 2020. PMID: 32939188 Free PMC article. Review. - Persisting viral sequences shape microbial CRISPR-based immunity.
Weinberger AD, Sun CL, Pluciński MM, Denef VJ, Thomas BC, Horvath P, Barrangou R, Gilmore MS, Getz WM, Banfield JF. Weinberger AD, et al. PLoS Comput Biol. 2012;8(4):e1002475. doi: 10.1371/journal.pcbi.1002475. Epub 2012 Apr 19. PLoS Comput Biol. 2012. PMID: 22532794 Free PMC article. - Evolution of animal Piwi-interacting RNAs and prokaryotic CRISPRs.
Kumar MS, Chen KC. Kumar MS, et al. Brief Funct Genomics. 2012 Jul;11(4):277-88. doi: 10.1093/bfgp/els016. Epub 2012 Apr 25. Brief Funct Genomics. 2012. PMID: 22539610 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases