Role of target geometry in phagocytosis - PubMed (original) (raw)
Role of target geometry in phagocytosis
Julie A Champion et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Phagocytosis is a principal component of the body's innate immunity in which macrophages internalize targets in an actin-dependent manner. Targets vary widely in shape and size and include particles such as pathogens and senescent cells. Despite considerable progress in understanding this complicated process, the role of target geometry in phagocytosis has remained elusive. Previous studies on phagocytosis have been performed using spherical targets, thereby overlooking the role of particle shape. Using polystyrene particles of various sizes and shapes, we studied phagocytosis by alveolar macrophages. We report a surprising finding that particle shape, not size, plays a dominant role in phagocytosis. All shapes were capable of initiating phagocytosis in at least one orientation. However, the local particle shape, measured by tangent angles, at the point of initial contact dictates whether macrophages initiate phagocytosis or simply spread on particles. The local shape determines the complexity of the actin structure that must be created to initiate phagocytosis and allow the membrane to move over the particle. Failure to create the required actin structure results in simple spreading and not internalization. Particle size primarily impacts the completion of phagocytosis in cases where particle volume exceeds the cell volume.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
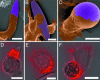
Fig. 1.
Scanning electron micrographs and 3D illustrations of PS particles created for phagocytosis experiments. (A) Spheres. (B) Oblate ellipsoids (13%). (C) Prolate ellipsoids (7%). (D) Elliptical disks (9%). (E) Rectangular disks (5%). (F) UFOs (12%). Particles are monodispersed with average standard deviations of measured dimensions for each shape listed in parentheses. A portion of this variation is due to 2–5% standard deviation in the diameter of spheres used as starting materials. (Scale bars: 5 μm.)
Fig. 2.
Time-lapse video microscopy clips spanning 39 min of macrophages interacting with identical nonopsonized ED particles (major axis 14 μm, minor axis 3 μm) from two different orientations. (A) Cell attaches along the major axis of an ED and internalizes it completely in 3 min. (B) Cell attaches to the flat side of an identical ED and spreads but does not internalize the particle. Continued observation indicated that this particle was not internalized for >110 min. (Scale bars: 10 μm.) (See Movies 1 and 2.) At least three cells were observed for each orientation of each particle type and size. Similar results were observed in all repetitions.
Fig. 3.
Scanning electron micrographs and actin staining confirm time-lapse video microscopy observations. Micrographs (A_–_C) of cells and particles were colored brown and purple, respectively. (A) The cell body can be seen at the end of an opsonized ED, and the membrane has progressed down the length of the particle. (Scale bar: 10 μm.) (B) A cell has attached to the flat side of an opsonized ED and has spread on the particle. (Scale bar: 5 μm.) (C) An opsonized spherical particle has attached to the top of a cell, and the membrane has progressed over approximately half the particle. (Scale bar: 5 μm.) (D_–_F) Overlays of bright-field and fluorescent images after fixing the cells and staining for polymerized actin with rhodamine phalloidin. (D) Actin ring forms as remodeling and depolymerization enable membrane to progress over an opsonized ED by new actin polymerization at the leading edge of the membrane. (E) Actin polymerization in the cell at site of attachment to flat side of an opsonized ED, but no actin cup or ring is visible. (F) Actin cup surrounds the end of an opsonized sphere as internalization begins after attachment. (Scale bars in D_–_F: 10 μm.) At least five cells were observed for each orientation of each particle. Similar results were observed in all repetitions.
Fig. 4.
Definition of Ω and its relationship with membrane velocity. (A) A schematic diagram illustrating how membrane progresses tangentially around an ED. T̄ represents the average of tangential angles from θ = 0 to θ = π/2. Ω is the angle between T̄ and membrane normal at the site of attachment, N̄. (B) Membrane velocity (distance traveled by the membrane divided by time to internalize, n ≥ 3; error bars represent SD) decreases with increasing Ω for a variety of shapes and sizes of particles. Nonopsonized particles are indicated by filled circles, and IgG-opsonized particles are indicated by open squares. Each data point represents a different shape, size, or aspect ratio particle. The internalization velocity is positive for Ω ≤ 45° (P < 0.001). Above a critical value of Ω, ≈45°, the internalization velocity is zero (P < 0.001) and there is only membrane spreading after particle attachment, not internalization. The arrows above the plot indicate the point of attachment for each shape that corresponds to the value of Ω on the x axis. Error in Ω is due to the difference in the actual point of contact in time-lapse microscopy from that used to calculate Ω. Only points of contact within 10° of that used to calculate Ω were selected. All data points at the critical point, Ω = 45°, except UFOs, do not have error associated with Ω because of their symmetry.
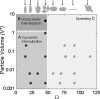
Fig. 5.
Phagocytosis phase diagram with Ω and dimensionless particle volume V* (particle volume divided by 7.5 μm radius spherical cell volume) as governing parameters (n = 5 for each point). Initialization of internalization is judged by the presence of an actin cup or ring as described in the text. There are three regions. Cells attaching to particles at areas of high Ω, >45°, spread but do not initiate internalization (region C). Cells attaching to particles at areas of low Ω, <45°, initiate internalization (regions A and B). If _V_* is ≤1, internalization is completed (region A). If _V_* > 1, internalization is not completed because of the size of the particle (region B). The arrows above the plot indicate the point of attachment for each shape that corresponds to the value of Ω on the x axis. Each case was classified as phagocytosis or no phagocytosis if >95% of observations were consistent. Each data point represents a different shape, size, or aspect ratio particle.
Similar articles
- Actin plays a crucial role in the phagocytosis and biological response to respirable quartz particles in macrophages.
Haberzettl P, Duffin R, Krämer U, Höhr D, Schins RP, Borm PJ, Albrecht C. Haberzettl P, et al. Arch Toxicol. 2007 Jul;81(7):459-70. doi: 10.1007/s00204-007-0178-5. Epub 2007 Mar 21. Arch Toxicol. 2007. PMID: 17375287 - Effect of shape and size of polymer particles on cellular internalization.
Park K. Park K. J Control Release. 2010 Nov 1;147(3):313. doi: 10.1016/j.jconrel.2010.10.001. Epub 2010 Oct 8. J Control Release. 2010. PMID: 20933025 No abstract available. - A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages.
Colucci-Guyon E, Niedergang F, Wallar BJ, Peng J, Alberts AS, Chavrier P. Colucci-Guyon E, et al. Curr Biol. 2005 Nov 22;15(22):2007-12. doi: 10.1016/j.cub.2005.09.051. Curr Biol. 2005. PMID: 16303559 - Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome.
Niedergang F, Chavrier P. Niedergang F, et al. Curr Opin Cell Biol. 2004 Aug;16(4):422-8. doi: 10.1016/j.ceb.2004.06.006. Curr Opin Cell Biol. 2004. PMID: 15261675 Review. - Picket-fences in the plasma membrane: functions in immune cells and phagocytosis.
Mylvaganam SM, Grinstein S, Freeman SA. Mylvaganam SM, et al. Semin Immunopathol. 2018 Nov;40(6):605-615. doi: 10.1007/s00281-018-0705-x. Epub 2018 Sep 12. Semin Immunopathol. 2018. PMID: 30209546 Review.
Cited by
- Nanoparticle shape improves delivery: rational coarse grain molecular dynamics (rCG-MD) of taxol in worm-like PEG-PCL micelles.
Loverde SM, Klein ML, Discher DE. Loverde SM, et al. Adv Mater. 2012 Jul 24;24(28):3823-30. doi: 10.1002/adma.201103192. Epub 2011 Nov 22. Adv Mater. 2012. PMID: 22105885 Free PMC article. - Immunostimulatory Polymers as Adjuvants, Immunotherapies, and Delivery Systems.
Weiss AM, Hossainy S, Rowan SJ, Hubbell JA, Esser-Kahn AP. Weiss AM, et al. Macromolecules. 2022 Aug 23;55(16):6913-6937. doi: 10.1021/acs.macromol.2c00854. Epub 2022 Aug 4. Macromolecules. 2022. PMID: 36034324 Free PMC article. Review. - The Impact of Aspect Ratio on the Biodistribution and Tumor Homing of Rigid Soft-Matter Nanorods.
Shukla S, Eber FJ, Nagarajan AS, DiFranco NA, Schmidt N, Wen AM, Eiben S, Twyman RM, Wege C, Steinmetz NF. Shukla S, et al. Adv Healthc Mater. 2015 Apr 22;4(6):874-82. doi: 10.1002/adhm.201400641. Epub 2015 Jan 13. Adv Healthc Mater. 2015. PMID: 25641794 Free PMC article. - Tuning innate immune activation by surface texturing of polymer microparticles: the role of shape in inflammasome activation.
Vaine CA, Patel MK, Zhu J, Lee E, Finberg RW, Hayward RC, Kurt-Jones EA. Vaine CA, et al. J Immunol. 2013 Apr 1;190(7):3525-32. doi: 10.4049/jimmunol.1200492. Epub 2013 Feb 20. J Immunol. 2013. PMID: 23427254 Free PMC article. - Engineering physical microenvironments to study innate immune cell biophysics.
Kalashnikov N, Moraes C. Kalashnikov N, et al. APL Bioeng. 2022 Sep 20;6(3):031504. doi: 10.1063/5.0098578. eCollection 2022 Sep. APL Bioeng. 2022. PMID: 36156981 Free PMC article. Review.
References
- Aderem A., Underhill D. M. Annu. Rev. Immunol. 1999;17:593–623. - PubMed
- Tabata Y., Ikada Y. Biomaterials. 1988;9:356–362. - PubMed
- Kawaguchi H., Koiwai N., Ohtsuka Y., Miyamoto M., Sasakawa S. Biomaterials. 1986;7:61–66. - PubMed
- Rudt S., Muller R. H. J. Controlled Release. 1992;22:263–271.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources