Evolution of four gene families with patchy phylogenetic distributions: influx of genes into protist genomes - PubMed (original) (raw)
Comparative Study
Evolution of four gene families with patchy phylogenetic distributions: influx of genes into protist genomes
Jan O Andersson et al. BMC Evol Biol. 2006.
Abstract
Background: Lateral gene transfer (LGT) in eukaryotes from non-organellar sources is a controversial subject in need of further study. Here we present gene distribution and phylogenetic analyses of the genes encoding the hybrid-cluster protein, A-type flavoprotein, glucosamine-6-phosphate isomerase, and alcohol dehydrogenase E. These four genes have a limited distribution among sequenced prokaryotic and eukaryotic genomes and were previously implicated in gene transfer events affecting eukaryotes. If our previous contention that these genes were introduced by LGT independently into the diplomonad and Entamoeba lineages were true, we expect that the number of putative transfers and the phylogenetic signal supporting LGT should be stable or increase, rather than decrease, when novel eukaryotic and prokaryotic homologs are added to the analyses.
Results: The addition of homologs from phagotrophic protists, including several Entamoeba species, the pelobiont Mastigamoeba balamuthi, and the parabasalid Trichomonas vaginalis, and a large quantity of sequences from genome projects resulted in an apparent increase in the number of putative transfer events affecting all three domains of life. Some of the eukaryotic transfers affect a wide range of protists, such as three divergent lineages of Amoebozoa, represented by Entamoeba, Mastigamoeba, and Dictyostelium, while other transfers only affect a limited diversity, for example only the Entamoeba lineage. These observations are consistent with a model where these genes have been introduced into protist genomes independently from various sources over a long evolutionary time.
Conclusion: Phylogenetic analyses of the updated datasets using more sophisticated phylogenetic methods, in combination with the gene distribution analyses, strengthened, rather than weakened, the support for LGT as an important mechanism affecting the evolution of these gene families. Thus, gene transfer seems to be an on-going evolutionary mechanism by which genes are spread between unrelated lineages of all three domains of life, further indicating the importance of LGT from non-organellar sources into eukaryotic genomes.
Figures
Figure 1
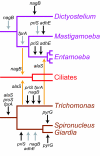
Distribution of the four genes in the taxa sampled in this study. A hypothetical tree of eukaryotes for which genomes have been fully sampled and published, *; is close to completion, **; or only partially sampled (genome sequence survey or expressed sequence tags), ***; indicating their classification into "super-groups" [29-31], showing the presence or absence of the four genes in the study. Please notice that the gene absences in the genomes that are close to completion are unconfirmed, they may turn into presences upon publication. A and B refer to strongly separated groups in the phylogenetic analyses, as indicated in Figures 2-4 & 6. The priS genes encode the hybrid-cluster proteins, fprA genes encode the A-type flavoproteins, nagB genes encode glucosamine-6-phosphate isomerase proteins and the adhE genes encode the alcohol dehydrogenase E proteins.
Figure 2
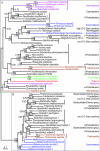
Protein maximum likelihood tree of hybrid-cluster protein (priS gene). ML tree based on 417 unambiguously aligned aa positions of the hybrid-cluster protein. Bootstrap support values >50% from ML analyses are shown above the branches. Posterior probabilities for the Bayesian consensus tree of the grouped aa analysis are shown below the branches. When no space is available a line indicates the position of the support values. Absence of a posterior probability value at a node indicates that this node was lacking in the Bayesian consensus tree. Details about the phylogenetic analyses are found in the Methods section and AdditionalAdditional File 2. The grey boxes A and B indicate strongly separated groups which include eukaryotic sequences. The tree is arbitrarily rooted. Eubacteria are labelled black, Archaea are labelled blue, and the Eukaryotes are labelled according to their classification into "super-groups" [29, 30]: opisthokonts (orange), amoebozoa (purple), chromalveolates (red), plants (green) and excavates (brown) (see Figure 1).
Figure 3
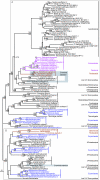
Protein maximum likelihood trees of A-type flavoprotein (fprA gene). ML trees based 269 unambiguously aligned aa positions of the A-type flavoprotein. The boxes indicate sequences that have an approximately 450 aa long conserved C-terminal extension of the flavoprotein which is absent from all other sequences in the alignment (see Additional File 4 for further analyses and discussion). The grey boxes A and B indicate strongly separated groups which include eukaryotic sequences. The tree is arbitrarily rooted. Details about the phylogenetic analyses are found in the Methods section and Additional File 2. Labelling as in Figure 2.
Figure 4
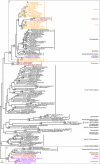
Protein maximum likelihood trees of the short and long versions of glucosamine-6-phosphate isomerase (nagB gene). ML tree based on 229 unambiguously aligned aa positions from the N-terminal part of the alignment of the glucosamine-6-phosphate isomerase protein. The grey boxes A and B indicate strongly separated groups which include eukaryotic sequences. The sequences in the B box (with the exception of the R. baltica 3 sequence) have an approximately 500 aa long conserved C-terminal extension of the protein which is absent from all other sequences in the alignment. The sequences in box B, together with the sequences indicated with asterisks were excluded in a separate analysis shown in Additional File 5, to test the influence of the removal of the long version of the protein and long branches on the relative positions of eukaryotic sequences. The tree is arbitrarily rooted. Details about the phylogenetic analyses are found in the Methods section and Additional File 2. Labelling as in Figure 2.
Figure 5
Protein maximum likelihood trees of the long version of glucosamine-6-phosphate isomerase (nagB gene). Phylogenetic tree based on 560 unambiguously aligned aa positions from the glucosamine-6-phosphate isomerase sequences that have the long C-terminal extension (box B in Figure 4). In a separate analysis the partial Mastigamoeba balamuthi sequence was included and its position is indicated with an arrow with the bootstrap support value in parenthesis. The tree is arbitrarily rooted. Details about the phylogenetic analyses are found in the Methods section and Additional File 2. Labelling as in Figure 2.
Figure 6
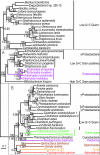
Protein maximum likelihood tree of alcohol dehydrogenase E (adhE gene). Phylogenetic tree based on 796 unambiguously aligned aa positions of the alcohol dehydrogenase E protein sequences. The grey boxes A and B indicate strongly separated groups which include eukaryotic sequences. The tree is arbitrarily rooted. Details about the phylogenetic analyses are found in the Methods section and Additional File 2. Labelling as in Figure 2.
Figure 7
Summary of putative lateral gene transfers affecting amoebozoa, ciliates, and diplomonads, and parabasalids. Lateral gene transfers inferred from Figures 2-6, as well as previously published phylogenetic analyses [10, 18] discussed in the text, are indicated on the topology; gene transfers from prokaryotes are indicated by black arrows, intra-eukaryote transfers between the groups are indicated by orange arrows, and gene introduced from uncertain origins are indicated by grey arrow. Please notice that the figure does not delineate the order of individual transfer events on each branch, and that plausible alternative hypotheses do exist to explain some of the unexpected phylogenetic positions of eukaryotes, here indicated as gene transfer events, our currently preferred hypothesis (see text for details).
Similar articles
- Evidence for lateral transfer of genes encoding ferredoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica.
Nixon JE, Wang A, Field J, Morrison HG, McArthur AG, Sogin ML, Loftus BJ, Samuelson J. Nixon JE, et al. Eukaryot Cell. 2002 Apr;1(2):181-90. doi: 10.1128/EC.1.2.181-190.2002. Eukaryot Cell. 2002. PMID: 12455953 Free PMC article. - Phylogenomic study indicates widespread lateral gene transfer in Entamoeba and suggests a past intimate relationship with parabasalids.
Grant JR, Katz LA. Grant JR, et al. Genome Biol Evol. 2014 Sep;6(9):2350-60. doi: 10.1093/gbe/evu179. Genome Biol Evol. 2014. PMID: 25146649 Free PMC article. - Phylogenetic analyses of diplomonad genes reveal frequent lateral gene transfers affecting eukaryotes.
Andersson JO, Sjögren AM, Davis LA, Embley TM, Roger AJ. Andersson JO, et al. Curr Biol. 2003 Jan 21;13(2):94-104. doi: 10.1016/s0960-9822(03)00003-4. Curr Biol. 2003. PMID: 12546782 - Lateral Gene Transfer Mechanisms and Pan-genomes in Eukaryotes.
Sibbald SJ, Eme L, Archibald JM, Roger AJ. Sibbald SJ, et al. Trends Parasitol. 2020 Nov;36(11):927-941. doi: 10.1016/j.pt.2020.07.014. Epub 2020 Aug 19. Trends Parasitol. 2020. PMID: 32828660 Review. - Too Much Eukaryote LGT.
Martin WF. Martin WF. Bioessays. 2017 Dec;39(12). doi: 10.1002/bies.201700115. Epub 2017 Oct 25. Bioessays. 2017. PMID: 29068466 Review.
Cited by
- Bioinformatics Structural and Phylogenetic Characterization of Entamoeba histolytica Alcohol Dehydrogenase 2 (EhADH2).
Lowerre KM, Espinosa A, Paz-Y-Miño-C G, Hemme C. Lowerre KM, et al. Bios. 2019 Oct;90(1):30-41. doi: 10.1893/0005-3155-90.1.30. Epub 2019 Oct 26. Bios. 2019. PMID: 34103738 Free PMC article. - Six-State Amino Acid Recoding is not an Effective Strategy to Offset Compositional Heterogeneity and Saturation in Phylogenetic Analyses.
Hernandez AM, Ryan JF. Hernandez AM, et al. Syst Biol. 2021 Oct 13;70(6):1200-1212. doi: 10.1093/sysbio/syab027. Syst Biol. 2021. PMID: 33837789 Free PMC article. - A molecular timescale for eukaryote evolution with implications for the origin of red algal-derived plastids.
Strassert JFH, Irisarri I, Williams TA, Burki F. Strassert JFH, et al. Nat Commun. 2021 Mar 25;12(1):1879. doi: 10.1038/s41467-021-22044-z. Nat Commun. 2021. PMID: 33767194 Free PMC article. - Identification of the NADH-oxidase gene in Trichomonas vaginalis.
Lamien-Meda A, Leitsch D. Lamien-Meda A, et al. Parasitol Res. 2020 Feb;119(2):683-686. doi: 10.1007/s00436-019-06572-8. Epub 2019 Dec 18. Parasitol Res. 2020. PMID: 31853623 Free PMC article. - Conservation of Atypical Allostery in C. elegans UDP-Glucose Dehydrogenase.
Beattie NR, Keul ND, Hicks Sirmans TN, McDonald WE, Talmadge TM, Taujale R, Kannan N, Wood ZA. Beattie NR, et al. ACS Omega. 2019 Sep 24;4(15):16318-16329. doi: 10.1021/acsomega.9b01565. eCollection 2019 Oct 8. ACS Omega. 2019. PMID: 31616809 Free PMC article.
References
- Loftus B, Anderson I, Davies R, Alsmark UCM, Samuelson J, Amedeo P, Roncaglia P, Berriman M, Hirt RP, Mann BJ, Nozaki T, Suh B, Pop M, Duchene M, Ackers J, Tannich E, Leippe M, Hofer M, Bruchhaus I, Willhoeft U, Bhattacharya A, Chillingworth T, Churcher C, Hance Z, Harris B, Harris D, Jagels K, Moule S, Mungall K, Ormond D, Squares R, Whitehead S, Quail MA, Rabbinowitsch E, Norbertczak H, Price C, Wang Z, Guillen N, Gilchrist C, Stroup SE, Bhattacharya S, Lohia A, Foster PG, Sicheritz-Ponten T, Weber C, Singh U, Mukherjee C, El-Sayed NM, Petri WAJ, Clark CG, Embley TM, Barrell B, Fraser CM, Hall N. The genome of the protist parasite Entamoeba histolytica. Nature. 2005;433:865–868. doi: 10.1038/nature03291. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources