Inflammatory responses to migrating Brugia pahangi third-stage larvae - PubMed (original) (raw)
Inflammatory responses to migrating Brugia pahangi third-stage larvae
Kristina H Porthouse et al. Infect Immun. 2006 Apr.
Abstract
Despite being central to parasite establishment and subsequent host pathological and immunologic responses, host-parasite interactions during early third-stage filarial larva (L3) migration are poorly understood. These studies aimed to define early tissue migration of Brugia pahangi L3 in the gerbil (Meriones unguiculatus) and measure host cellular responses during this period. Gerbils were intradermally inoculated in the hind limb with 100 B. pahangi L3, and necropsies were performed at various times. At 3 h, most L3 (96.3%) were recovered from tissues associated with the infection site, with marked L3 migration occurring by 24 h. Larvae were dispersed throughout the lymphatics at 7 days postinfection (dpi), and at 28 dpi, most parasites were recovered from the spermatic cord lymphatics. Parasites were identified histologically at all time points. Inflammatory cells, primarily neutrophils, were frequently observed around larvae in the dermis and muscle near the injection site at 3 h and 24 h. Levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha mRNA peaked at 3 h in all tissues, with IL-6 levels also high in the spleen at 28 dpi. Levels of IL-4 mRNA were elevated in all tissues at 28 dpi. These observations demonstrate that L3 migrate quickly through various tissues and into lymph nodes in a predictable pattern. Migrating L3 induce an early acute inflammatory response that is modulated as parasites establish in the lymphatics. Polarization of the host response towards a dominant Th2-like profile is present at 7 dpi and is well established by 28 dpi in this permissive host.
Figures
FIG. 1.
Histologic sections of Brugia pahangi L3 in various tissues during early migration. The bars shown indicate a size of 50 μm. (A) Larvae in the dermis of the left hind limb of a gerbil 3 h after i.d. infection with 100 B. pahangi L3. The arrow indicates inflammatory infiltrate surrounding the larvae that consists primarily of neutrophils with lesser numbers of eosinophils, basophils, and mononuclear cells. (B) Brugia pahangi larvae within a deep dermal lymphatic vessel of a gerbil 3 days after i.d. infection with 100 B. pahangi L3. Arrows indicate larvae within the lymphatic vessel. (C) Larvae in the subcapsular sinus of the left popliteal lymph node of a gerbil 7 days after i.d. infection with 100 B. pahangi L3. Arrows indicate larvae within the nodal sinus. No significant inflammation was apparent within the lymph node.
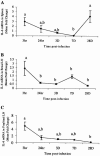
FIG. 2.
Quantitation of IL-6 mRNA in spleens (A), renal lymph nodes (LN) (B), and popliteal lymph nodes (C). Gerbils were necropsied at 3 h, 24 h, 3 days, 7 days, and 28 days after i.d. inoculation with Brugia pahangi L3. mRNA levels were measured by reverse transcription (RT)-PCR, and values are expressed as mean severalfold changes compared with values from control animals. Superscript letters indicate statistical significance (P < 0.05). Values with the same letter are not statistically different.
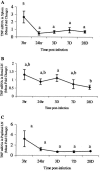
FIG. 3.
Quantitation of TNF-α mRNA in spleens (A), renal lymph nodes (LN) (B), and popliteal lymph nodes (C). Gerbils were necropsied at 3 h, 24 h, 3 days, 7 days, and 28 days after i.d. inoculation with Brugia pahangi L3. mRNA levels were measured by RT-PCR, and values are expressed as the mean severalfold changes compared with values from control animals. Superscript letters indicate statistical significance (P < 0.05). Values with the same letter are not statistically different. No statistically significant differences were found in the spleen (P = 0.065) or popliteal lymph node (P = 0.2).
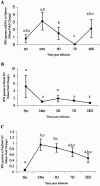
FIG. 4.
Quantitation of IFN-γ mRNA in spleens (A), renal lymph nodes (LN) (B), and popliteal lymph nodes (C). Gerbils were necropsied at 3 h, 24 h, 3 days, 7 days, and 28 days after i.d. inoculation with B. pahangi L3. mRNA levels were measured by RT-PCR, and values are expressed as mean severalfold changes compared with values from control animals. Superscript letters indicate statistical significance (P < 0.05). Values with the same letter are not statistically different. No statistically significant differences were found in the renal lymph nodes (P = 0.094).
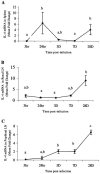
FIG. 5.
Quantitation of IL-4 mRNA in spleens (A), renal lymph nodes (LN) (B), and popliteal lymph nodes (C). Gerbils were necropsied at 3 h, 24 h, 3 days, 7 days, and 28 days after i.d. inoculation with B. pahangi L3. mRNA levels were measured by RT-PCR, and values are expressed as the mean severalfold changes compared with values from control animals. Superscript letters indicate statistical significance (P < 0.05). Values with the same letter are not statistically different.
Similar articles
- Brugia pahangi: immunization with early L3 ES alters parasite migration, and reduces microfilaremia and lymphatic lesion formation in gerbils (Meriones unguiculatus).
Zipperer GR, Arumugam S, Chirgwin SR, Coleman SU, Shakya KP, Klei TR. Zipperer GR, et al. Exp Parasitol. 2013 Oct;135(2):446-55. doi: 10.1016/j.exppara.2013.08.007. Epub 2013 Aug 24. Exp Parasitol. 2013. PMID: 23981910 Free PMC article. - Effect of immunostimulatory oligodeoxynucleotides on host responses and the establishment of Brugia pahangi in Mongolian gerbils (Meriones unguiculatus).
Chirgwin SR, Nowling JM, Coleman SU, Klei TR. Chirgwin SR, et al. J Parasitol. 2003 Jun;89(3):483-9. doi: 10.1645/GE-3088. J Parasitol. 2003. PMID: 12880245 - Cellular immune responses of jirds to extracts of life cycle stages and adult excretory secretory products during the early development of Brugia pahangi.
Rao UR, Nasarre C, Coleman SU, Bakeer M, Dennis VA, Horohov DW, Klei TR. Rao UR, et al. Exp Parasitol. 1996 Apr;82(3):255-66. doi: 10.1006/expr.1996.0033. Exp Parasitol. 1996. PMID: 8631377 - Tissue migration capability of larval and adult Brugia pahangi.
Chirgwin SR, Coleman SU, Porthouse KH, Klei TR. Chirgwin SR, et al. J Parasitol. 2006 Feb;92(1):46-51. doi: 10.1645/GE-599R.1. J Parasitol. 2006. PMID: 16629314 - Ocular Brugia pahangi Filariasis Complicated by Severe Macular Damage in Thailand: Case Report and Literature Review.
Suphap N, Somkijrungroj T, Kongwattananon W, Supawatjariyakul W, Pataradool T, Kraivichian K, Jantarabenjakul W, Tulvatana W, Preativatanyou K. Suphap N, et al. Am J Trop Med Hyg. 2024 Apr 30;110(6):1158-1164. doi: 10.4269/ajtmh.24-0047. Print 2024 Jun 5. Am J Trop Med Hyg. 2024. PMID: 38688273 Free PMC article. Review.
Cited by
- Effect of Brugia pahangi co-infection with Plasmodium berghei ANKA in gerbils (Meriones unguiculatus).
Junaid OQ, Vythilingam I, Khaw LT, Sivanandam S, Mahmud R. Junaid OQ, et al. Parasitol Res. 2020 Apr;119(4):1301-1315. doi: 10.1007/s00436-020-06632-4. Epub 2020 Mar 16. Parasitol Res. 2020. PMID: 32179986 - Dose-Dependent Prophylactic Efficacy of Filarial Antigens Glutathione-S-Transferase and Abundant Larval Transcript-2 against Brugia malayi Challenge in Mastomys.
Nakhale MR, Bhoj P, Togre N, Khatri V, Batra L, Padigel U, Goswami K. Nakhale MR, et al. Can J Infect Dis Med Microbiol. 2024 Jul 26;2024:4543922. doi: 10.1155/2024/4543922. eCollection 2024. Can J Infect Dis Med Microbiol. 2024. PMID: 39105125 Free PMC article. - S100A8/S100A9 deficiency increases neutrophil activation and protective immune responses against invading infective L3 larvae of the filarial nematode Litomosoides sigmodontis.
Frohberger SJ, Fercoq F, Neumann AL, Surendar J, Stamminger W, Ehrens A, Karunakaran I, Remion E, Vogl T, Hoerauf A, Martin C, Hübner MP. Frohberger SJ, et al. PLoS Negl Trop Dis. 2020 Feb 27;14(2):e0008119. doi: 10.1371/journal.pntd.0008119. eCollection 2020 Feb. PLoS Negl Trop Dis. 2020. PMID: 32107497 Free PMC article. - Attempts to Image the Early Inflammatory Response during Infection with the Lymphatic Filarial Nematode Brugia pahangi in a Mouse Model.
Myburgh E, Ritchie R, Goundry A, O'Neill K, Marchesi F, Devaney E. Myburgh E, et al. PLoS One. 2016 Dec 16;11(12):e0168602. doi: 10.1371/journal.pone.0168602. eCollection 2016. PLoS One. 2016. PMID: 27992545 Free PMC article.
References
- Ah, H. S., T. R. Klei, J. W. McCall, and P. E. Thompson. 1974. Brugia pahangi infections in Mongolian jirds and dogs following the ocular inoculation of infective larvae. J. Parasitol. 60:643-648. - PubMed
- Reference deleted.
- Ah, H. S., and P. E. Thompson. 1973. Brugia pahangi: infections and their effect on the lymphatic system of Mongolian jirds (Meriones unguiculatus). Exp. Parasitol. 34:393-411. - PubMed
- Ash, L. R. 1973. Chronic Brugia pahangi and Brugia malayi infections in Meriones unguiculatus. J. Parasitol. 59:442-447. - PubMed
- Ash, L. R., and J. M. Riley. 1970. Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus, with notes on infections in other rodents. J. Parasitol. 56:969-973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources