MyD88 innate immune function in a zebrafish embryo infection model - PubMed (original) (raw)
MyD88 innate immune function in a zebrafish embryo infection model
Astrid M van der Sar et al. Infect Immun. 2006 Apr.
Abstract
Innate immunity signaling mechanisms during vertebrate embryogenesis are largely unknown. To study Toll-like receptor (TLR) signaling function in the zebrafish embryo model, we designed an experimental setup for antisense morpholino knockdown under conditions of bacterial infection. Clearance of Salmonella enterica serovar Typhimurium Ra bacteria was significantly impaired after knockdown of myeloid differentiation factor 88 (MyD88), a common adaptor protein in TLR and interleukin-1 receptor signaling. Thereby, we demonstrate for the first time that the innate immune response of the developing embryo involves MyD88-dependent signaling, which further establishes the zebrafish embryo as a model for the study of vertebrate innate immunity.
Figures
FIG. 1.
RT-PCR analysis of zebrafish TLR and adaptor genes at different developmental stages. β-Actin (βACT) expression was determined for reference. One hundred nanograms of total RNA was used in the RT-PCR mixtures, except for zTRIF and βACT, where 50 ng was used. Forty cycles of amplification were used in all cases. The timing of development of the zebrafish immune system is indicated with marked arrow heads: S, hematopoietic stem cells can be distinguished in the ventrolateral mesoderm; M, embryonic macrophages migrate over the yolk sac, enter blood circulation, and are able to phagocytose injected bacteria; G, cells with typical granulocyte morphology can be distinguished that are able to localize to sites of acute inflammation; L, immature lymphoblasts can be detected and myelopoiesis is taken over by the anterior kidney; A, adaptive immunity is matured after 4 to 6 weeks of development.
FIG. 2.
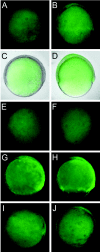
Specificity of the MyD88 and 5-bp mismatch control morpholino. Embryos were injected with 2 ng of MyD88 morpholino (A, C, E, G, I) or with 2 ng of 5-bp mismatch control morpholino (B, D, F, H, J). (A, B) Coinjection of MyD88 (A) or mismatch (B) morpholino with 2 pg of _zMyD88_-GFP mRNA, which includes the 5′ leader sequence at which the morpholino is targeted. (C, D) Overlay of the fluorescence images from panels A and B with bright-field images of the same embryos. Note the absence of green fluorescent protein (GFP) signal in the embryonic tissues of the embryo coinjected with the MyD88 morpholino (A, C) and the presence of GFP signal in the embryonic tissues of the embryo coinjected with the mismatch control morpholino (B, D). The yolk shows autofluorescence independent of injection of the GFP construct. (E, F) Control embryos injected with MyD88 (E) or mismatch (F) morpholinos only, showing similar yolk autofluorescence as the embryo in panel A. (G, H) Coinjection of MyD88 (G) or mismatch (H) morpholino with 2 pg of GFP mRNA. (I, J) Coinjection of MyD88 (I) or mismatch (J) morpholino with 2 pg of a modified _zMyD88_-GFP mRNA lacking the morpholino target site. Note that fluorescence in the embryonic tissues of the embryos shown in panels G to J is unaffected by injection of the different morpholinos. Fluorescence images were acquired with a Leica DC500 camera and MZ Fluo 3 stereomicroscope. Fluorescence recordings were made with a fixed exposure time of 10.4 s and with the gain set at 1. Contrast was enhanced by 70% during image processing with Adobe Photoshop 6.0.
FIG. 3.
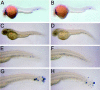
Development and properties of myeloid cells in MyD88 morphants. Embryos injected with 1.7 ng of MyD88 morpholino (A, C, E, G) or with 1.7 ng of 5-bp mismatch control morpholino (B, D, F, H) were analyzed for L-plastin expression in macrophages (A, B) and for myeloperoxidase activity in granulocytes (C to H). (A, B), 1 dpf embryos; (C, D), 2 dpf embryos; (E, F) tails of the embryos shown in panels C and D; (G, H) tails of 2-dpf embryos analyzed 6 h after wounding of the tail fin. Embryos were grown in 0.003% 1-phenyl-2-thiourea (Sigma) to prevent melanization. Composite images were made of different focal planes.
FIG. 4.
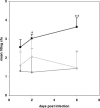
Number of CFU of S. enterica serovar Typhimurium Ra isolated from infected wild-type (⧫), mismatch (▴), and MyD88 morphant (▪) embryos at different time points (dpi). Groups of 5 embryos were analyzed at each time point, and the mean 10log CFU value are presented on the graph. The numbers are the averages of results from four independent experiments. Statistical analyses were performed with single-factor analysis of variance tests and indicated that the difference between total CFU in wild-type and morphant embryos was significant at P values of <0.05 (*) at 2 dpi and <0.01 (**) at 6 dpi. The difference between total CFU in mismatch and morphant embryos was also significant at a P value of <0.01 (**) at 6 dpi.
FIG. 5.
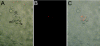
Presence of S. enterica serovar Typhimurium Ra bacteria inside macrophages of a MyD88 morphant embryo. MyD88 morphant embryos were infected at 28 hpf by injection of DsRed-expressing S. enterica serovar Typhimurium Ra bacteria into the axial vein, and images of infected macrophages in the yolk sac circulation valley were taken after 1 h using a Leica DC500 camera and MZ 16 FA microscope. (A) Bright-field image showing a group of macrophages (m) and erythrocytes (e). (B) Fluorescence image of S. enterica serovar Typhimurium Ra in the same location as the macrophages. (C) Overlay image of panels A and B, indicating the ability of macrophages of MyD88 morphants to phagocytose bacteria. Scale bar, 10 μm.
References
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. - PubMed
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
- Bennett, C. M., J. P. Kanki, J. Rhodes, T. X. Liu, B. H. Paw, M. W. Kieran, D. M. Langenau, A. Delahaye-Brown, L. I. Zon, M. D. Fleming, and A. T. Look. 2001. Myelopoiesis in the zebrafish, Danio rerio. Blood 98:643-651. - PubMed
- Crowhurst, M. O., J. E. Layton, and G. J. Lieschke. 2002. Developmental biology of zebrafish myeloid cells. Int. J. Dev. Biol. 46:483-492. - PubMed
- Davidson, A. J., and L. I. Zon. 2004. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 23:7233-7246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases