Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration - PubMed (original) (raw)
Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration
Brad A Grueter et al. J Neurosci. 2006.
Abstract
The bed nucleus of the stria terminalis (BNST) is a key component of the CNS stress and reward circuit. Synaptic plasticity in this region could in part underlie the persistent behavioral alterations in generalized anxiety and addiction. Group I metabotropic glutamate receptors (mGluRs) have been implicated in stress, addiction, and synaptic plasticity, but their roles in the BNST are unknown. We find that activation of group I mGluRs in the dorsal BNST induces depression of excitatory synaptic transmission through two distinct mechanisms. First, a combined activation of group I mGluRs (mGluR1 and mGluR5) induces a transient depression that is cannabinoid 1 receptor dependent. Second, as with endocannabinoid-independent group I mGluR long-term depression (LTD) in the adult hippocampus, we find that activation of mGluR5 induces an extracellular signal-regulated kinase (ERK)-dependent LTD. Surprisingly, our data demonstrate that this LTD requires the ERK1 rather than ERK2 isoform, establishing a key role for this isoform in the CNS. Finally, we find that this LTD is dramatically reduced after multiple exposures but not a single exposure to cocaine, suggesting a role for this form of plasticity in the actions of psychostimulants on anxiety and reward circuitries and their emergent control of animal behavior.
Figures
Figure 1.
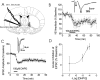
Group 1 mGluR activation in the BNST results in LTD of excitatory transmission. A, Diagram of coronal mouse brain containing the BNST (shaded in gray). B, Application of DHPG-induced a persistent reduction in the synaptic response (N2), as measured by the peak amplitude of field potential responses, normalized to the average value during the basal period before drug application. Inset, Sample traces from one experiment before (dark line) and after (light line) DHPG application, average of six consecutive traces at 18–20 and 38–40 min. Error bars indicate SEM. C, Time course of DHPG-induced reduction in EPSC amplitude, normalized to the average value during the basal period before drug application. Inset, Representative EPSC traces from BNST neurons before and after DHPG application, average of 10 consecutive traces at 4–5 and 12–13 min. D, Concentration–response curve of peak DHPG-induced depression of EPSCs (n = 3–12).
Figure 2.
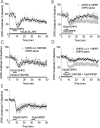
DHPG induces a dual-component persistent depression in the BNST. A, The NMDA receptor antagonist,
dl
-AP-5 (100 μ
m
) does not alter DHPG-induced depression of EPSCs (n = 3). B, MPEP blocks the late but not the peak effect of DHPG on EPSCs (n = 3). C, DHPG effects on BNST EPSCs are unchanged in the presence of LY367385 (n = 3). D, Both the mGluR1 antagonist LY367385 and MPEP are required to attenuate the peak DHPG effect on EPSCs (n = 5). E, Late MPEP application does not reverse DHPG-induced LTD (n = 3). Error bars indicate SE.
Figure 3.
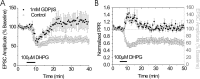
Characterization of DHPG-induced LTD. A, Blockade of postsynaptic G-protein signaling with GDPβS prevents DHPG-induced depression of EPSCs (filled squares; n = 5) but not control cells containing GTP (open circles). B, Time course of normalized PPR illustrating transient increase in release probability in response to DHPG (left axis). As illustrated, DHPG causes a transient increase in PPR during the peak effect on EPSC amplitude but returns to basal levels during the LTD (filled squares, PPR; open circles, EPSC amplitude). Error bars indicate SE.
Figure 4.
The early component of DHPG-induced depression of the EPSC is CB1R dependent but the LTD is CB1R independent. A, Summary of the effects of DHPG (100 μ
m
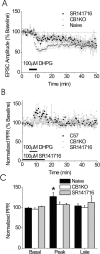
) on EPSCs in BNST from CB1R knock-out (KO) mice (white circles; n = 9) in the presence of SR141716 (black squares; n = 3) and control mice (gray triangles; n = 12). B, The PPR is unchanged in the CB1R knock-out or in the presence of SR141716 after DHPG application. C, Bar graph representing the basal, peak, and late effects of DHPG on PPR in control CB1R knock-out mice and in the presence of SR141716 (n = 9 and 12, respectively; *p < 0.05 compared with basal). Error bars indicate SE.
Figure 5.
mGluR5-dependent LTD in the BNST is ERK dependent. A, DHPG-induced LTD of EPSCs is abolished by the presence of the MEK inhibitor U0126 (20 μ
m
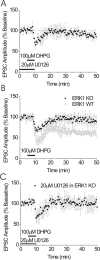
; n = 6). U0126 was applied for a minimum of 30 min before DHPG application. B, EPSCs from BNST neurons from ERK1 knock-out (KO) mice do not elicit LTD after DHPG application (n = 6). WT, Wild type. Inset represents presence/absence of ERK1 protein in tissue punches containing BNST in control and ERK1 knock-out mice. Error bars indicate SE.
Figure 6.
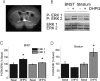
DHPG application results in phosphorylation of ERK. A, Representative slice illustrating size and location of BNST and striatal punches taken for biochemical analysis. B, Representative immunoblot for phosphorylated ERK1/ERK2 and pan ERK1/ERK2 from either the BNST or the striatum in the absence or presence of DHPG. DHPG (100 μ
m
) was applied for 5 min, after which tissue punches were immediately collected. C, D, Quantitative analysis of ERK1 and ERK2 phosphorylation in the BNST (C; n = 8; *p < 0.05) and the striatum (D; n = 8; *p < 0.05). Error bars indicate SE.
Figure 7.
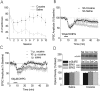
mGluR5-dependent LTD is attenuated by contingent and noncontingent in vivo cocaine. A, Summary of active lever presses for cocaine pressing versus saline pressing mice (*p < 0.05). B, Summary of the effects of cocaine self-administration on mGluR5-induced LTD in the BNST (cocaine, n = 9; saline, n = 13). C, Summary of the effects of intraperitoneal administration of cocaine on mGluR-induced LTD in the BNST (repeated intraperitoneal cocaine, gray triangles, n = 8; single intraperitoneal cocaine, black squares, n = 6; saline, white circles, n = 8). D, Quantification of total levels of mGluR5, ERK1, and ERK2 protein from BNST punches obtained from chronically treated (intraperitoneally) mice (saline, n = 14; cocaine, n = 12). Error bars indicate SE.
Similar articles
- In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression.
Grueter BA, McElligott ZA, Robison AJ, Mathews GC, Winder DG. Grueter BA, et al. J Neurosci. 2008 Sep 10;28(37):9261-70. doi: 10.1523/JNEUROSCI.2886-08.2008. J Neurosci. 2008. PMID: 18784306 Free PMC article. - Alpha1-adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders.
McElligott ZA, Winder DG. McElligott ZA, et al. Neuropsychopharmacology. 2008 Sep;33(10):2313-23. doi: 10.1038/sj.npp.1301635. Epub 2007 Nov 28. Neuropsychopharmacology. 2008. PMID: 18046308 Free PMC article. - Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis.
Grueter BA, Winder DG. Grueter BA, et al. Neuropsychopharmacology. 2005 Jul;30(7):1302-11. doi: 10.1038/sj.npp.1300672. Neuropsychopharmacology. 2005. PMID: 15812571 - Extracellular signal-regulated protein kinases 1 and 2 activation by addictive drugs: a signal toward pathological adaptation.
Pascoli V, Cahill E, Bellivier F, Caboche J, Vanhoutte P. Pascoli V, et al. Biol Psychiatry. 2014 Dec 15;76(12):917-26. doi: 10.1016/j.biopsych.2014.04.005. Epub 2014 Apr 18. Biol Psychiatry. 2014. PMID: 24844603 Review. - Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis.
McElligott ZA, Winder DG. McElligott ZA, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Nov 13;33(8):1329-35. doi: 10.1016/j.pnpbp.2009.05.022. Epub 2009 Jun 11. Prog Neuropsychopharmacol Biol Psychiatry. 2009. PMID: 19524008 Free PMC article. Review.
Cited by
- Endocannabinoids Released in the Ventral Tegmental Area During Copulation to Satiety Modulate Changes in Glutamate Receptors Associated With Synaptic Plasticity Processes.
Rodríguez-Manzo G, González-Morales E, Garduño-Gutiérrez R. Rodríguez-Manzo G, et al. Front Synaptic Neurosci. 2021 Aug 18;13:701290. doi: 10.3389/fnsyn.2021.701290. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34483875 Free PMC article. - Endocannabinoids at the synapse and beyond: implications for neuropsychiatric disease pathophysiology and treatment.
Scheyer A, Yasmin F, Naskar S, Patel S. Scheyer A, et al. Neuropsychopharmacology. 2023 Jan;48(1):37-53. doi: 10.1038/s41386-022-01438-7. Epub 2022 Sep 13. Neuropsychopharmacology. 2023. PMID: 36100658 Free PMC article. Review. - Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects.
Pan B, Zhong P, Sun D, Liu QS. Pan B, et al. J Neurosci. 2011 Aug 3;31(31):11244-55. doi: 10.1523/JNEUROSCI.1040-11.2011. J Neurosci. 2011. PMID: 21813685 Free PMC article. - mGluR-Dependent Synaptic Plasticity in Drug-Seeking.
Bellone C, Mameli M. Bellone C, et al. Front Pharmacol. 2012 Aug 27;3:159. doi: 10.3389/fphar.2012.00159. eCollection 2012. Front Pharmacol. 2012. PMID: 22969723 Free PMC article. - Cocaine and Amphetamine Induce Overlapping but Distinct Patterns of AMPAR Plasticity in Nucleus Accumbens Medium Spiny Neurons.
Jedynak J, Hearing M, Ingebretson A, Ebner SR, Kelly M, Fischer RA, Kourrich S, Thomas MJ. Jedynak J, et al. Neuropsychopharmacology. 2016 Jan;41(2):464-76. doi: 10.1038/npp.2015.168. Epub 2015 Jun 12. Neuropsychopharmacology. 2016. PMID: 26068728 Free PMC article.
References
- Adams JP, Sweatt JD (2002). Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42:135–163. - PubMed
- Adwanikar H, Karim F, Gereau RW IV (2004). Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain 111:125–135. - PubMed
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y (1999). The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci 877:486–498. - PubMed
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH (2001). MAP kinases. Chem Rev 101:2449–2476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous