A voltage-gated proton-selective channel lacking the pore domain - PubMed (original) (raw)
. 2006 Apr 27;440(7088):1213-6.
doi: 10.1038/nature04700. Epub 2006 Mar 22.
Affiliations
- PMID: 16554753
- PMCID: PMC4084761
- DOI: 10.1038/nature04700
A voltage-gated proton-selective channel lacking the pore domain
I Scott Ramsey et al. Nature. 2006.
Abstract
Voltage changes across the cell membrane control the gating of many cation-selective ion channels. Conserved from bacteria to humans, the voltage-gated-ligand superfamily of ion channels are encoded as polypeptide chains of six transmembrane-spanning segments (S1-S6). S1-S4 functions as a self-contained voltage-sensing domain (VSD), in essence a positively charged lever that moves in response to voltage changes. The VSD 'ligand' transmits force via a linker to the S5-S6 pore domain 'receptor', thereby opening or closing the channel. The ascidian VSD protein Ci-VSP gates a phosphatase activity rather than a channel pore, indicating that VSDs function independently of ion channels. Here we describe a mammalian VSD protein (H(V)1) that lacks a discernible pore domain but is sufficient for expression of a voltage-sensitive proton-selective ion channel activity. H(v)1 currents are activated at depolarizing voltages, sensitive to the transmembrane pH gradient, H+-selective, and Zn2+-sensitive. Mutagenesis of H(v)1 identified three arginine residues in S4 that regulate channel gating and two histidine residues that are required for extracellular inhibition of H(v)1 by Zn2+. H(v)1 is expressed in immune tissues and manifests the characteristic properties of native proton conductances (G(vH+)). In phagocytic leukocytes, G(vH+) are required to support the oxidative burst that underlies microbial killing by the innate immune system. The data presented here identify H(v)1 as a long-sought voltage-gated H+ channel and establish H(v)1 as the founding member of a family of mammalian VSD proteins.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
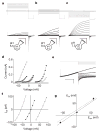
Figure 1. Biophysical properties of expressed Hv1 currents
Depolarizing voltage steps (5 mV increments) were applied to an _Hv1_-transfected HM1 cell (a–c) to elicit outward H+ currents and deactivating inward tail currents (−80 mV). TMA5.5o, TMA6.5o or TMA7.5o (TMA6.5i for all) were used to impose pH gradients indicated in the diagrams. a, ρHi/o+=0.1, _V_h = −40 mV (_V_step = +30 mV to +80 mV, scale bar 0.2 nA, 1 s; b, ρHi/o+=1, _V_h = −40 mV (_V_step = −30 mV to +20 mV), scale bar 0.1 nA, 1 s; c, ρHi/o+=10, _V_h = −70 mV (_V_step = −60 mV to −10 mV), scale bar 0.2 nA, 1 s. d, Hv1 currents at the end of the depolarizing step (_I_step, open symbols) and the absolute value of _I_tail (−80 mV, filled symbols) are plotted as a function of the step voltage. Data shown are from the cell shown in a–c. Circles, ρHi/o+=0.1 (_V_thr = 70 ± 5.8 mV, n = 3); triangles, ρHi/o+=1.0 (_V_thr = 11.7 ± 2.1 mV, n = 6); squares, ρHi/o+=10 (_V_thr = −31.7 ± 3.3 mV, n = 3). e, Representative currents for _E_rev measurement (_V_h = −40 mV, _V_step = +40 mV, _V_tail = −100 mV to +40 mV). Small _I_step (<200 pA) was chosen to minimize Hi+ depletion (note constant outward current level). f, Monoexponential fits of _I_tail were extrapolated to t = 0 and _E_rev was estimated from the zero-current intercept of linear fits to the data. Open circles, TMA6.5i, TMA5.5o (ρHi/o+=0.1); filled triangles, TMA6.5i, TMA6.5o (ρHi/o+=1); open squares, TMA6.5i, TMA7.5o (ρHi/o+=10). Average _E_rev values: _E_rev = 53.3 ± 1.4 mV, ρHi/o+=0.1, n = 10; _E_rev = 0.9 ± 0.7 mV, ρHi/o+=1.0, n = 14; _E_rev = −56.5 ± 2.6 mV, ρHi/o+=10, n = 5. Data represent mean ± s.e.m. from n experiments. g, _V_thr is plotted against the Nernst potential for H+ at 24 °C. The data are fitted to _V_thr = 0.82 _E_rev + 13.8 mV (solid line). Data represent mean ± s.e.m. from n = 3–7 experiments. The _V_thr versus _E_rev relationship for native _G_vH+ (_V_thr = 0.79 _E_rev + 23 mV, dotted line) is shown for comparison.
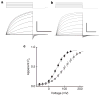
Figure 2. Hv1 voltage-dependent gating
a, Hv1 currents (−60 mV to +120 mV, _V_h = −40 mV, ρHi/o+=10, Na6.5i, Na7.5o) in a representative cell. Scale bar 2 nA, 400 ms. Under symmetrical conditions (TMA6.5, ρHi/o+=1), _τ_act = 715 ± 124 ms (+80 mV), n = 9 and _τ_deact = 65.0 ± 12.1 ms (−80 mV), n = 7. b, R205A (−60 mV to +180 mV, _V_h = −60 mV, ρHi/o+=10, Na6.5i, Na7.5o). Scale bar 2 nA, 10 ms. Note the 40-fold difference in timescale compared to a. c, The I_tail–_V relation (−80 mV, ρHi/o+=10, Na6.5i, Na7.5o) was normalized to the maximum current obtained from a Boltzmann fit to the data for each cell expressing Hv1 (filled circles) or R205A (open circles) to estimate _P_o − V. Data were fitted to a Boltzmann function (solid lines) and normalized to the extrapolated maximum current. Points represent mean ± s.e.m. of normalized data. A comparison of curve fits from individual experiments (Hv1, _V_0.5 = 58.0 ± 5.6 mV, zδ = 0.90 ± 0.04, n = 6; R205A, _V_0.5 = 99.5 ± 18.3 mV, zδ = 0.57* ± 0.03, n = 3; *P = 0.03 by Student’s non-paired _t_-test) indicates that significantly less effective charge is moved in R205A than in Hv1 during channel gating. Data represent mean ± s.e.m. from n experiments.
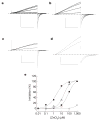
Figure 3. Mutations in Hv1 reveal residues required for Zn2+ inhibition
Inhibition of _I_step (+25 mV to +50 mV) in cells superfused with EGTA-free TMA6.5 or Na6.5 (ρHi/o+=1) was typically faster than the sampling interval (10 s) and washout was always complete. Average IC50 = 2.2 ± 0.6 μM, _n_H = 1.0 ± 0.2, n = 4; ρHi/o+=1, + 40 mV, TMA6.5. Data represent mean ± s.e.m. from n experiments. The applied voltages and [Zn2+] for the records shown (Na6.5, ρHi/o+=1) were: a, Hv1, _V_step = +40/_V_tail = −80 mV, [Zn2+] = 0, 0.1, 1, 10, 100 μM; b, H140A, +40/−60 mV, [Zn2+] = 0, 1, 10, 100 μM; c, H193A, +30/−80 mV, [Zn2+] = 0, 10, 100 μM; d, H140A/H193A, +50/−20 mV, [Zn2+] = 0, 1 mM. Scale bars: 100 pA, 1 s. e, Representative Zn2+ concentration-response curves for Hv1 (filled squares, IC50 = 1.9 μM, _n_H = 0.9), H193A (open triangles, IC50 = 17.9 μM, _n_H = 1.2), H140A (filled circles, IC50 = 74.3 μM, _n_H = 1.0), and H140A/H193A (open squares, maximum inhibition = 11.1 ± 3.4%, n = 3).
Similar articles
- Hydrophobic gasket mutation produces gating pore currents in closed human voltage-gated proton channels.
Banh R, Cherny VV, Morgan D, Musset B, Thomas S, Kulleperuma K, Smith SME, Pomès R, DeCoursey TE. Banh R, et al. Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):18951-18961. doi: 10.1073/pnas.1905462116. Epub 2019 Aug 28. Proc Natl Acad Sci U S A. 2019. PMID: 31462498 Free PMC article. - Voltage-gated proton (H(v)1) channels, a singular voltage sensing domain.
Castillo K, Pupo A, Baez-Nieto D, Contreras GF, Morera FJ, Neely A, Latorre R, Gonzalez C. Castillo K, et al. FEBS Lett. 2015 Nov 14;589(22):3471-8. doi: 10.1016/j.febslet.2015.08.003. Epub 2015 Aug 18. FEBS Lett. 2015. PMID: 26296320 Review. - Molecular biology and biophysical properties of ion channel gating pores.
Moreau A, Gosselin-Badaroudine P, Chahine M. Moreau A, et al. Q Rev Biophys. 2014 Nov;47(4):364-88. doi: 10.1017/S0033583514000109. Q Rev Biophys. 2014. PMID: 25382261 Review. - The Role of Proton Transport in Gating Current in a Voltage Gated Ion Channel, as Shown by Quantum Calculations.
Kariev AM, Green ME. Kariev AM, et al. Sensors (Basel). 2018 Sep 18;18(9):3143. doi: 10.3390/s18093143. Sensors (Basel). 2018. PMID: 30231473 Free PMC article. - Dimer interaction in the Hv1 proton channel.
Mony L, Stroebel D, Isacoff EY. Mony L, et al. Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20898-20907. doi: 10.1073/pnas.2010032117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788354 Free PMC article.
Cited by
- A specialized molecular motion opens the Hv1 voltage-gated proton channel.
Mony L, Berger TK, Isacoff EY. Mony L, et al. Nat Struct Mol Biol. 2015 Apr;22(4):283-290. doi: 10.1038/nsmb.2978. Epub 2015 Mar 2. Nat Struct Mol Biol. 2015. PMID: 25730777 Free PMC article. - Ion channel associated diseases: overview of molecular mechanisms.
Zaydman MA, Silva JR, Cui J. Zaydman MA, et al. Chem Rev. 2012 Dec 12;112(12):6319-33. doi: 10.1021/cr300360k. Epub 2012 Nov 14. Chem Rev. 2012. PMID: 23151230 Free PMC article. Review. No abstract available. - Temperature Dependent Activity of the Voltage-Gated Proton Channel.
Fujiwara Y. Fujiwara Y. Adv Exp Med Biol. 2024;1461:109-125. doi: 10.1007/978-981-97-4584-5_8. Adv Exp Med Biol. 2024. PMID: 39289277 Review. - Solution structure and phospholipid interactions of the isolated voltage-sensor domain from KvAP.
Butterwick JA, MacKinnon R. Butterwick JA, et al. J Mol Biol. 2010 Nov 5;403(4):591-606. doi: 10.1016/j.jmb.2010.09.012. Epub 2010 Sep 21. J Mol Biol. 2010. PMID: 20851706 Free PMC article. - Ion channel voltage sensors: structure, function, and pathophysiology.
Catterall WA. Catterall WA. Neuron. 2010 Sep 23;67(6):915-28. doi: 10.1016/j.neuron.2010.08.021. Neuron. 2010. PMID: 20869590 Free PMC article. Review.
References
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. - PubMed
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. - PubMed
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. - PubMed
- DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. - PubMed
- DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases