Applying FSL to the FIAC data: model-based and model-free analysis of voice and sentence repetition priming - PubMed (original) (raw)
Applying FSL to the FIAC data: model-based and model-free analysis of voice and sentence repetition priming
Christian F Beckmann et al. Hum Brain Mapp. 2006 May.
Abstract
This article presents results obtained from applying various tools from FSL (FMRIB Software Library) to data from the repetition priming experiment used for the HBM'05 Functional Image Analysis Contest. We present analyses from the model-based General Linear Model (GLM) tool (FEAT) and from the model-free independent component analysis tool (MELODIC). We also discuss the application of tools for the correction of image distortions prior to the statistical analysis and the utility of recent advances in functional magnetic resonance imaging (FMRI) time series modeling and inference such as the use of optimal constrained HRF basis function modeling and mixture modeling inference. The combination of hemodynamic response function (HRF) and mixture modeling, in particular, revealed that both sentence content and speaker voice priming effects occurred bilaterally along the length of the superior temporal sulcus (STS). These results suggest that both are processed in a single underlying system without any significant asymmetries for content vs. voice processing.
Figures
Figure 1
Setup of prestatistical processing steps in the FEAT GUI.
Figure 2
First‐level design matrices used for the model‐based (GLM) analysis. The columns of the design matrices show graphical representation of the GLM regressors. For the FIAC data there were 4 regressors modeling the BOLD changes during the 4 experimental conditions, together with temporal derivatives (each regressor is immediately followed by its temporal derivative), which are used to account for misspecification of the hemodynamic lag. The design matrix plots also show the width of the temporal high‐pass filter (far left; any low‐frequency fluctuation with periodicity larger than the indicated red bar is removed from the data). At the bottom are the contrast vectors used for testing different primary or differential BOLD changes.
Figure 3
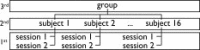
Hierarchical multilevel GLM approach for mixed‐effects analysis of the FIAC data: at the first level, each individual session's data is analysed using first‐level designs described in Figure 2. At the intermediate level, each subject's different sessions are combined into mixed‐effects subject‐specific means. At the third (top) level, these subject means are then used to calculate mixed‐effects group mean estimates.
Figure 4
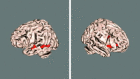
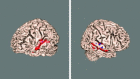
Cross‐subject mean (contrast 4: different sentences different speakers). Results are shown for the block‐design data (blue) and the event‐related design (red). All 2‐D image slices are shown left‐right reversed. 3‐D rendering carried out using FSLView.
Figure 5
Repetition effect of speakers (contrast 5: SStSSp < SStDSp) estimated from the event‐related datasets (red) when sentence content is not changed between different speakers. No supra‐thresholded voxels were found in the block‐design data.
Figure 6
Repetition effect of sentences (contrast 9: SStDSp < DStDSp) estimated from the block‐design data (blue) and the event‐related data sets (red). No supra‐thresholded voxels were found in the right hemisphere.
Figure 7
Effect of the cognitive interaction: maximum effect of repetition suppression (contrast 13: SStSSp < DStDSp).
Figure 8
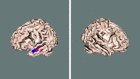
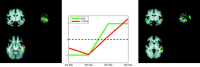
Estimated speaker effect when changing sentence content (contrast 6: DStSSp < DStDSp) from the event‐related data. No significant difference was found when null‐hypothesis testing with Gaussian field theory was used for inference. When using alternative hypothesis testing via mixture modeling (_P_ > 0.5), however, some difference was found, shown here in blue. Even more significant voxels were reported when, in addition, the HRF modeling was enhanced through the use of constrained optimal linear basis sets (shown in red).
Figure 9
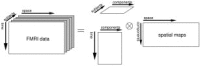
Schematic illustration of the model‐free multisubject tensor‐ICA approach: the data are represented as a time × space × subject block of data that are then decomposed as the outer product of matrices describing the different signal components in the spatial, temporal, and subject domains.
Figure 10
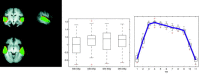
Results from the tensor‐ICA decompositions of the block‐design data. For the spatial map DStDSp, red/yellow = tensor‐ICA, blue = GLM, green = both. The tensor‐ICA spatial maps were thresholded by transforming the spatial raw IC estimates into _Z_‐statistic maps (dividing raw IC estimates by the voxel‐wise residual standard error of the decomposition) and thresholding these values at a posterior probability level of “activation” (P > 0.5) based on a Gaussian/Gamma mixture model fitted to the distribution of spatial Z values.
Figure 11
“Contrasts” of primary tensor‐ICA mean effects maps: (A) contrast 13 (SStSSp < DStDSp) calculated explicitly from the 2 primary tensor‐ICA maps (red‐yellow) together with contrast 13 from the GLM analysis (green); (B) “exploratory contrast” calculated by decomposing the 4 primary auditory maps from the initial tensor‐ICA decompositions using standard ICA. One of the resulting columns of the mixing matrix shows strong correlation with the standard GLM contrast 10, and the spatial map (C; red‐yellow) is shown on top of GLM mixed‐effects results for contrast 10 (C; green).
Figure 12
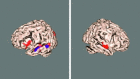
(A) Additional group maps, depicting structured BOLD fluctuations in functionally relevant cortical areas, such as medial visual cortex (yellow), lateral visual cortical areas (red), visual stream (green), motor (pink), and visuospatial attention (blue), often termed the default‐mode network [Gusnard and Raichle, 2001]. (B) Estimated temporal responses within the default‐mode network for the 4 different conditions (gray, shown inverted) together with the temporal response for the primary activation pattern (black; average over all 4 conditions).
Similar articles
- Functional imaging analysis contest (FIAC) analysis according to AFNI and SUMA.
Saad ZS, Chen G, Reynolds RC, Christidis PP, Hammett KR, Bellgowan PS, Cox RW. Saad ZS, et al. Hum Brain Mapp. 2006 May;27(5):417-24. doi: 10.1002/hbm.20247. Hum Brain Mapp. 2006. PMID: 16568421 Free PMC article. - Permutation testing of orthogonal factorial effects in a language-processing experiment using fMRI.
Suckling J, Davis MH, Ooi C, Wink AM, Fadili J, Salvador R, Welchew D, Sendur L, Maxim V, Bullmore ET. Suckling J, et al. Hum Brain Mapp. 2006 May;27(5):425-33. doi: 10.1002/hbm.20252. Hum Brain Mapp. 2006. PMID: 16596618 Free PMC article. - Neuroimaging of syntax and syntactic processing.
Grodzinsky Y, Friederici AD. Grodzinsky Y, et al. Curr Opin Neurobiol. 2006 Apr;16(2):240-6. doi: 10.1016/j.conb.2006.03.007. Epub 2006 Mar 24. Curr Opin Neurobiol. 2006. PMID: 16563739 Review. - Functional MRI of language: new approaches to understanding the cortical organization of semantic processing.
Bookheimer S. Bookheimer S. Annu Rev Neurosci. 2002;25:151-88. doi: 10.1146/annurev.neuro.25.112701.142946. Epub 2002 Mar 19. Annu Rev Neurosci. 2002. PMID: 12052907 Review.
Cited by
- Model-Based and Model-Free Analyses of the Neural Correlates of Tongue Movements.
Sörös P, Schäfer S, Witt K. Sörös P, et al. Front Neurosci. 2020 Mar 24;14:226. doi: 10.3389/fnins.2020.00226. eCollection 2020. Front Neurosci. 2020. PMID: 32265635 Free PMC article. - Alterations in the Brain Structure and Functional Connectivity in Aquaporin-4 Antibody-Positive Neuromyelitis Optica Spectrum Disorder.
Yan J, Wang Y, Miao H, Kwapong WR, Lu Y, Ma Q, Chen W, Tu Y, Liu X. Yan J, et al. Front Neurosci. 2020 Jan 14;13:1362. doi: 10.3389/fnins.2019.01362. eCollection 2019. Front Neurosci. 2020. PMID: 32009872 Free PMC article. - Cerebellar neural markers of susceptibility to social isolation and positive affective processing.
Wong NML, Shao R, Wu J, Tao J, Chen L, Lee TMC. Wong NML, et al. Brain Struct Funct. 2019 Dec;224(9):3339-3351. doi: 10.1007/s00429-019-01965-y. Epub 2019 Nov 7. Brain Struct Funct. 2019. PMID: 31701265 Free PMC article. - Autobiographical memory and default mode network function in schizophrenia: an fMRI study.
Martin-Subero M, Fuentes-Claramonte P, Salgado-Pineda P, Salavert J, Arevalo A, Bosque C, Sarri C, Guerrero-Pedraza A, Santo-Angles A, Capdevila A, Sarró S, Salvador R, McKenna PJ, Pomarol-Clotet E. Martin-Subero M, et al. Psychol Med. 2021 Jan;51(1):121-128. doi: 10.1017/S0033291719003052. Epub 2019 Nov 4. Psychol Med. 2021. PMID: 31680659 Free PMC article. - Neuroepigenetic signatures of age and sex in the living human brain.
Gilbert TM, Zürcher NR, Catanese MC, Tseng CJ, Di Biase MA, Lyall AE, Hightower BG, Parmar AJ, Bhanot A, Wu CJ, Hibert ML, Kim M, Mahmood U, Stufflebeam SM, Schroeder FA, Wang C, Roffman JL, Holt DJ, Greve DN, Pasternak O, Kubicki M, Wey HY, Hooker JM. Gilbert TM, et al. Nat Commun. 2019 Jul 3;10(1):2945. doi: 10.1038/s41467-019-11031-0. Nat Commun. 2019. PMID: 31270332 Free PMC article.
References
- Beckmann CF, Smith SM (2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging, 23: 137–152. - PubMed
- Beckmann CF, Smith SM (2005): Tensorial extensions of independent component analysis for multisubject FMRI analysis. NeuroImage 25: 294–311. - PubMed
- Beckmann CF, Jenkinson M, Smith SM (2003a): General multi‐level linear modelling for group analysis in FMRI. Neuroimage 20: 1052–1063. - PubMed
- Beckmann CF, Woolrich M, Smith SM (2003b): Gaussian/gamma mixture modelling of ICA/GLM spatial maps. NeuroImage 19: S985.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical