Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons - PubMed (original) (raw)
Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons
Michael L Molineux et al. Proc Natl Acad Sci U S A. 2006.
Abstract
T-type calcium channels are thought to transform neuronal output to a burst mode by generating low voltage-activated (LVA) calcium currents and rebound burst discharge. In this study we assess the expression pattern of the three different T-type channel isoforms (Ca(v)3.1, Ca(v)3.2, and Ca(v)3.3) in cerebellar neurons and focus on their potential role in generating LVA spikes and rebound discharge in deep cerebellar nuclear (DCN) neurons. We detected expression of one or more Ca(v)3 channel isoforms in a wide range of cerebellar neurons and selective expression of different isoforms in DCN cells. We further identify two classes of large-diameter DCN neurons that exhibit either a strong or weak capability for rebound discharge, despite the ability to generate LVA spikes when calcium currents are pharmacologically isolated. By correlating the Ca(v)3 channel expression pattern with the electrophysiological profile of identified DCN cells, we show that Ca(v)3.1 channels are expressed in isolation in DCN-burst cells, whereas Ca(v)3.3 is expressed in DCN-weak burst cells. Ca(v)3.1-expressing DCN cells correspond to excitatory or GABAergic neurons, whereas Ca(v)3.3-expressing cells are non-GABAergic. The Ca(v)3 class of LVA calcium channels is thus expressed in specific combinations in a wide range of cerebellar neurons but contributes to rebound burst discharge in only a select number of cell classes.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
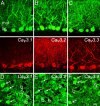
Fig. 1.
Cav3.1, Cav3.2, and Cav3.3 immunolabel of cerebellar output neurons. (A_–_C) Images of cerebellar Purkinje cells double-labeled for calbindin are paired with images of Cav3 immunolabel. mol, molecular; PC, Purkinje cell. (D_–_F) Cav3.1, Cav3.2, and Cav3.3 labeling of DCN neurons. A diffuse interstitial label allows one to distinguish a heterogeneous Cav3 expression pattern in DCN neurons. Filled arrows indicate Cav3-positive neurons and open arrows indicate DCN neurons negative for a given Cav3 immunolabel. (Scale bar: 20 μm.)
Fig. 2.
Cav3 isoform expression in cerebellar interneurons. Cerebellar interneuron image pairs of Cav3.1, Cav3.2, or Cav3.3 immunolabel are shown juxtaposed to microtubule-associated protein-2-labeled cell bodies and dendrites. Labeling of Golgi cells (A, D, and G), stellate cells (B, E, and H), and basket cells (C, F, and I) is shown. The structural immunolabel in F is calbindin, revealing basket cells as negative images against a background of Purkinje cell dendrites. Filled arrows denote cells positive for immunolabel, and open arrows indicate those negative for Cav3 immunolabel. (Scale bars: 20 μm.)
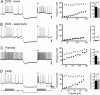
Fig. 3.
Cerebellar cells exhibit different degrees of rebound burst discharge. Shown are in vitro recordings and FI plots calculated at the symbols indicated above the recordings. Na+ spike discharge was evoked by a set of 300-ms test pulses, delivered first from resting potential and then after a 500-ms hyperpolarization from −80 to −90 mV. The current protocol is shown in the lowest trace, and break marks indicate a 4.5-s recovery period between step commands. Histogram plots of the mean frequency change over the FI plots are shown at right under control conditions (black bars) and in 2 mM Cs+ to block _I_h (gray bars). Large-diameter DCN cells exhibit either a strong burst (A) or weak burst (B). By comparison, Purkinje cells (C) and Golgi cells (D) exhibit a moderate rebound discharge. _I_h contributes to rebound bursts only in Purkinje cells.
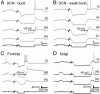
Fig. 4.
Bursting and weak-bursting cerebellar cell types can exhibit isolated LVA calcium spikes. Representative recordings from the indicated cerebellar cell types are shown in normal artificial cerebrospinal fluid with sodium spike discharge intact (i), after addition of Tetrodotoxin (TTX) and Cs+ (ii), or TTX, Cs+, tetraethylammonium, and 4-aminopyridine (iii). The dashed lines denote the detected voltage threshold for the LVA calcium spike. (iv) Final addition of 1 mM Ni2+ eliminates the calcium spike response in all cases. The current injection protocol is shown below each set of recordings. (Scale bars: 200 ms and 20 mV, A, B, and D; 500 ms and 20 mV, C.)
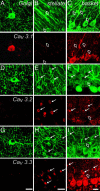
Fig. 5.
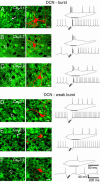
DCN-burst and DCN-weak-burst neurons are selectively labeled by Cav3 channel isoforms. Shown are representative cases of DCN-burst (A_–_C) and DCN-weak burst cells (D_–_F). (Left and Center) Immunolabels for each Cav3 isoform (Left) and the associated neurobiotin-filled cell (Center). (Right) Recordings from each filled cell with an expanded inset of the burst. (A_–_C) DCN-burst neurons are positive for Cav3.1 (A, solid arrow; n = 8) but negative for Cav3.3 (B, n = 5) and Cav3.2 (C, n = 5) (open arrows). (D_–_F) DCN-weak bursting neurons are positive for Cav3.3 (E, n = 4) but negative for Cav3.1 (D, n = 6) and Cav3.2 (F, n = 6). Cav3 labeling was identified in a 1- to 2-μm section through the soma of the recorded cell. Neurobiotin fills are stacked sections of up to 60-μm depth superimposed on the Cav3 image. (Scale bars: 20 μm.)
Fig. 6.
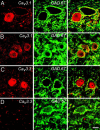
Differential expression of Cav3 isoforms in GAD67-positive DCN neurons. Images of DCN cells double-labeled for GAD and either Cav3.1 or Cav3.3. Shown is immunolabel for Cav3 channels (Left), GAD (Center), and the combined images (Right). (A and B) DCN cells positive for Cav3.1 can be either negative (A) or positive (B) for GAD immunolabel. (C and D) Cav3.3 label is found in GAD-negative neurons (C) but not in GAD-positive cells (D). (Scale bar: 20 μm.)
Similar articles
- Ca(V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons.
McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW. McKay BE, et al. Eur J Neurosci. 2006 Nov;24(9):2581-94. doi: 10.1111/j.1460-9568.2006.05136.x. Eur J Neurosci. 2006. PMID: 17100846 - Ionic factors governing rebound burst phenotype in rat deep cerebellar neurons.
Molineux ML, Mehaffey WH, Tadayonnejad R, Anderson D, Tennent AF, Turner RW. Molineux ML, et al. J Neurophysiol. 2008 Nov;100(5):2684-701. doi: 10.1152/jn.90427.2008. Epub 2008 Sep 3. J Neurophysiol. 2008. PMID: 18768644 - Contrasting the effects of nifedipine on subtypes of endogenous and recombinant T-type Ca2+ channels.
Shcheglovitov A, Zhelay T, Vitko Y, Osipenko V, Perez-Reyes E, Kostyuk P, Shuba Y. Shcheglovitov A, et al. Biochem Pharmacol. 2005 Mar 1;69(5):841-54. doi: 10.1016/j.bcp.2004.11.024. Epub 2005 Jan 12. Biochem Pharmacol. 2005. PMID: 15710361 - Voltage-dependent calcium channels.
Lacinová L. Lacinová L. Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review. - Molecular physiology of low-voltage-activated t-type calcium channels.
Perez-Reyes E. Perez-Reyes E. Physiol Rev. 2003 Jan;83(1):117-61. doi: 10.1152/physrev.00018.2002. Physiol Rev. 2003. PMID: 12506128 Review.
Cited by
- Low threshold T-type calcium channels as targets for novel epilepsy treatments.
Powell KL, Cain SM, Snutch TP, O'Brien TJ. Powell KL, et al. Br J Clin Pharmacol. 2014 May;77(5):729-39. doi: 10.1111/bcp.12205. Br J Clin Pharmacol. 2014. PMID: 23834404 Free PMC article. Review. - Rescue of motor coordination by Purkinje cell-targeted restoration of Kv3.3 channels in Kcnc3-null mice requires Kcnc1.
Hurlock EC, Bose M, Pierce G, Joho RH. Hurlock EC, et al. J Neurosci. 2009 Dec 16;29(50):15735-44. doi: 10.1523/JNEUROSCI.4048-09.2009. J Neurosci. 2009. PMID: 20016089 Free PMC article. - T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor.
Handforth A, Homanics GE, Covey DF, Krishnan K, Lee JY, Sakimura K, Martin FC, Quesada A. Handforth A, et al. Neuropharmacology. 2010 Nov;59(6):380-7. doi: 10.1016/j.neuropharm.2010.05.012. Epub 2010 Jun 12. Neuropharmacology. 2010. PMID: 20547167 Free PMC article. - Lock-and-key mechanisms of cerebellar memory recall based on rebound currents.
Wetmore DZ, Mukamel EA, Schnitzer MJ. Wetmore DZ, et al. J Neurophysiol. 2008 Oct;100(4):2328-47. doi: 10.1152/jn.00344.2007. Epub 2007 Aug 1. J Neurophysiol. 2008. PMID: 17671105 Free PMC article.
References
- Huguenard J. R. Annu. Rev. Physiol. 1996;58:329–348. - PubMed
- Perez-Reyes E., Cribbs L. L., Daud A., Lacerda A. E., Barclay J., Williamson M. P., Fox M., Rees M., Lee J. H. Nature. 1998;391:896–900. - PubMed
- Williams M. E., Washburn M. S., Hans M., Urrutia A., Brust P. F., Prodanovich P., Harpold M. M., Stauderman K. A. J. Neurochem. 1999;72:791–799. - PubMed
- McRory J. E., Santi C. M., Hamming K. S., Mezeyova J., Sutton K. G., Baillie D. L., Stea A., Snutch T. P. J. Biol. Chem. 2001;276:3999–4011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous