Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease - PubMed (original) (raw)
Review
Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease
Robert W Mahley et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The premise of this review is that apolipoprotein (apo) E4 is much more than a contributing factor to neurodegeneration. ApoE has critical functions in redistributing lipids among CNS cells for normal lipid homeostasis, repairing injured neurons, maintaining synapto-dendritic connections, and scavenging toxins. In multiple pathways affecting neuropathology, including Alzheimer's disease, apoE acts directly or in concert with age, head injury, oxidative stress, ischemia, inflammation, and excess amyloid beta peptide production to cause neurological disorders, accelerating progression, altering prognosis, or lowering age of onset. We envision that unique structural features of apoE4 are responsible for apoE4-associated neuropathology. Although the structures of apoE2, apoE3, and apoE4 are in dynamic equilibrium, apoE4, which is detrimental in a variety of neurological disorders, is more likely to assume a pathological conformation. Importantly, apoE4 displays domain interaction (an interaction between the N- and C-terminal domains of the protein that results in a compact structure) and molten globule formation (the formation of stable, reactive intermediates with potentially pathological activities). In response to CNS stress or injury, neurons can synthesize apoE. ApoE4 uniquely undergoes neuron-specific proteolysis, resulting in bioactive toxic fragments that enter the cytosol, alter the cytoskeleton, disrupt mitochondrial energy balance, and cause cell death. Our findings suggest potential therapeutic strategies, including the use of "structure correctors" to convert apoE4 to an "apoE3-like" molecule, protease inhibitors to prevent the generation of toxic apoE4 fragments, and "mitochondrial protectors" to prevent cellular energy disruption.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
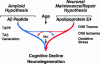
Fig. 1.
Multiple factors interact through various pathways to cause cognitive decline and neurodegeneration. ApoE4 may be associated with neuropathology through interactions with Aβ peptide that converge on the amyloid cascade or may act independently of Aβ.
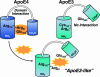
Fig. 2.
ApoE4 domain interaction can be disrupted by small molecules (represented by gold symbol). In apoE4, Arg-61 in the N-terminal domain interacts with Glu-255 in the C-terminal domain. Small molecules that are predicted to interact with apoE4 in the region of Arg-61 would disrupt domain interaction and convert apoE4 to an “apoE3-like” molecule.
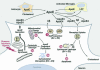
Fig. 3.
Roles of apoE in lipid redistribution among cells in the CNS and apoE isoform-specific differences in neuropathology. ApoE is synthesized by astrocytes, activated microglia, and neurons. Three potential detrimental roles for apoE4: (1) enhanced Aβ production, (2) potentiation of Aβ1–42-induced lysosomal leakage and apoptosis, and (3) enhanced neuron-specific proteolysis resulting in translocation of neurotoxic apoE4 fragments in the cytosol, where they are associated with cytoskeletal disruption and mitochondrial dysfunction.
Fig. 4.
Regions of apoE4 involved in causing neurotoxicity, translocation into the cytosol, and mitochondrial targeting. The receptor binding region (amino acids 136–150) plays a key role in translocation, as well as in binding to the LDL receptor and tau. The lipid binding region (amino acids 240–270) plays a role in translocation and mitochondrial localization, in addition to possessing lipid and Aβ binding.
Comment in
- Profile of Robert W. Mahley.
Nuzzo R. Nuzzo R. Proc Natl Acad Sci U S A. 2006 Apr 11;103(15):5641-3. doi: 10.1073/pnas.0601981103. Epub 2006 Apr 3. Proc Natl Acad Sci U S A. 2006. PMID: 16585500 Free PMC article. No abstract available.
Similar articles
- Apolipoprotein (apo) E4 and Alzheimer's disease: unique conformational and biophysical properties of apoE4 can modulate neuropathology.
Mahley RW, Huang Y. Mahley RW, et al. Acta Neurol Scand Suppl. 2006;185:8-14. doi: 10.1111/j.1600-0404.2006.00679.x. Acta Neurol Scand Suppl. 2006. PMID: 16866905 Review. - Plasma ApoE4 Levels Are Lower than ApoE2 and ApoE3 Levels, and Not Associated with Plasma Aβ40/42 Ratio as a Biomarker of Amyloid-β Amyloidosis in Alzheimer's Disease.
Nakamura T, Kawarabayashi T, Ueda T, Shimomura S, Hoshino M, Itoh K, Ihara K, Nakaji S, Takatama M, Ikeda Y, Shoji M. Nakamura T, et al. J Alzheimers Dis. 2023;93(1):333-348. doi: 10.3233/JAD-220996. J Alzheimers Dis. 2023. PMID: 36970894 - Apolipoprotein e sets the stage: response to injury triggers neuropathology.
Mahley RW, Huang Y. Mahley RW, et al. Neuron. 2012 Dec 6;76(5):871-85. doi: 10.1016/j.neuron.2012.11.020. Neuron. 2012. PMID: 23217737 Free PMC article. Review. - Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease.
Butterfield DA, Mattson MP. Butterfield DA, et al. Neurobiol Dis. 2020 May;138:104795. doi: 10.1016/j.nbd.2020.104795. Epub 2020 Feb 6. Neurobiol Dis. 2020. PMID: 32036033 Free PMC article. Review. - Interaction of nascent ApoE2, ApoE3, and ApoE4 isoforms expressed in mammalian cells with amyloid peptide beta (1-40). Relevance to Alzheimer's disease.
Aleshkov S, Abraham CR, Zannis VI. Aleshkov S, et al. Biochemistry. 1997 Aug 26;36(34):10571-80. doi: 10.1021/bi9626362. Biochemistry. 1997. PMID: 9265639
Cited by
- A Dietary Treatment Improves Cerebral Blood Flow and Brain Connectivity in Aging apoE4 Mice.
Wiesmann M, Zerbi V, Jansen D, Haast R, Lütjohann D, Broersen LM, Heerschap A, Kiliaan AJ. Wiesmann M, et al. Neural Plast. 2016;2016:6846721. doi: 10.1155/2016/6846721. Epub 2016 Mar 10. Neural Plast. 2016. PMID: 27034849 Free PMC article. - Altered Energy Metabolism Pathways in the Posterior Cingulate in Young Adult Apolipoprotein E ɛ4 Carriers.
Perkins M, Wolf AB, Chavira B, Shonebarger D, Meckel JP, Leung L, Ballina L, Ly S, Saini A, Jones TB, Vallejo J, Jentarra G, Valla J. Perkins M, et al. J Alzheimers Dis. 2016 Apr 23;53(1):95-106. doi: 10.3233/JAD-151205. J Alzheimers Dis. 2016. PMID: 27128370 Free PMC article. - Exercise Intervention for Alzheimer's Disease: Unraveling Neurobiological Mechanisms and Assessing Effects.
Ren J, Xiao H. Ren J, et al. Life (Basel). 2023 Nov 30;13(12):2285. doi: 10.3390/life13122285. Life (Basel). 2023. PMID: 38137886 Free PMC article. Review. - Design and Validation of Liposomal ApoE2 Gene Delivery System to Evade Blood-Brain Barrier for Effective Treatment of Alzheimer's Disease.
Arora S, Layek B, Singh J. Arora S, et al. Mol Pharm. 2021 Feb 1;18(2):714-725. doi: 10.1021/acs.molpharmaceut.0c00461. Epub 2020 Sep 16. Mol Pharm. 2021. PMID: 32787268 Free PMC article. - Small molecule structure correctors abolish detrimental effects of apolipoprotein E4 in cultured neurons.
Chen HK, Liu Z, Meyer-Franke A, Brodbeck J, Miranda RD, McGuire JG, Pleiss MA, Ji ZS, Balestra ME, Walker DW, Xu Q, Jeong DE, Budamagunta MS, Voss JC, Freedman SB, Weisgraber KH, Huang Y, Mahley RW. Chen HK, et al. J Biol Chem. 2012 Feb 17;287(8):5253-66. doi: 10.1074/jbc.M111.276162. Epub 2011 Dec 12. J Biol Chem. 2012. PMID: 22158868 Free PMC article.
References
- Mahley R. W. Science. 1988;240:622–630. - PubMed
- Weisgraber K. H. Adv. Protein Chem. 1994;45:249–302. - PubMed
- Mahley R. W., Rall S. C., Jr. Annu. Rev. Genomics Hum. Genet. 2000;1:507–537. - PubMed
- Corder E. H., Saunders A. M., Risch N. J., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Jr., Rimmler J. B., Locke P. A., Conneally P. M., Schmader K. E., et al. Nat. Genet. 1994;7:180–184. - PubMed
- Lippa C. F., Smith T. W., Saunders A. M., Hulette C., Pulaski-Salo D., Roses A. D. Neurology. 1997;48:515–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous