The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease - PubMed (original) (raw)
. 2006 Apr 4;103(14):5466-71.
doi: 10.1073/pnas.0509694103. Epub 2006 Mar 27.
Noel S Murcia, Claire H Larson, Seng Hui Low, Ryan Hedgepeth, Nicole Brown, Chris A Flask, Andrew C Novick, David A Goldfarb, Albrecht Kramer-Zucker, Gerd Walz, Klaus B Piontek, Gregory G Germino, Thomas Weimbs
Affiliations
- PMID: 16567633
- PMCID: PMC1459378
- DOI: 10.1073/pnas.0509694103
The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease
Jonathan M Shillingford et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Autosomal-dominant polycystic kidney disease (ADPKD) is a common genetic disorder that frequently leads to renal failure. Mutations in polycystin-1 (PC1) underlie most cases of ADPKD, but the function of PC1 has remained poorly understood. No preventive treatment for this disease is available. Here, we show that the cytoplasmic tail of PC1 interacts with tuberin, and the mTOR pathway is inappropriately activated in cyst-lining epithelial cells in human ADPKD patients and mouse models. Rapamycin, an inhibitor of mTOR, is highly effective in reducing renal cystogenesis in two independent mouse models of PKD. Treatment of human ADPKD transplant-recipient patients with rapamycin results in a significant reduction in native polycystic kidney size. These results indicate that PC1 has an important function in the regulation of the mTOR pathway and that this pathway provides a target for medical therapy of ADPKD.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
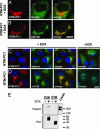
Fig. 1.
The N-terminal cytoplasmic domain of PC1 (NTM-PC1) colocalizes with mTOR and colocalizes and interacts with tuberin. (A_–_D) Expression of NTM-PC1 was induced with DOX for 16 h before fixation and immunostaining with antibodies against the indicated proteins. Noninduced NTM-PC1 cells served as controls (C and D, −DOX). Grp94 and GM130 are resident proteins of the endoplasmic reticulum and Golgi apparatus, respectively. (E) FLM-PC1 and CTM-PC1 cells were cultured in the absence or presence of DOX for 16 h, the PC1 fusion proteins were immunoprecipitated by using an antibody against CD16, and binding proteins were analyzed by Western blot with the indicated antibodies. The starting material (MDCK lysate) serves as a control. [Scale bars, 3 μm (A_–_D).]
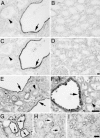
Fig. 2.
Phospho-mTOR, -S6K, and -S6 are specifically induced in cyst-lining epithelial cells in ADPKD and mouse models. (A_–_D) Renal tissue sections isolated from human ADPKD patients (A and C) or normal patients (B and D) were analyzed by immunohistochemistry using antibodies against phospho-mTOR (Ser 2448) (A and B) or phospho-S6K (Thr 389) (C and D). Note specific staining of cystic epithelial cells (A and C, arrows) but not surrounding normal epithelium (A and C, arrowheads). (E_–_I) Renal tissue sections derived from Pkd1cond/cond:TgN(balancer1)2Cgn (E) and MAL-overexpressing (F) mice, nontreated (G) or rapamycin-treated (H) (5 mg/kg of body weight) _oprk_-rescue (Tg737orpk/orpk;TgRsq) mutant mice, and nontreated _orpk_-heterozygous-rescue (Tg737orpk/+;TgRsq) (I) control mice were immunostained by using an antibody against phospho-S6 ribosomal protein (Ser 235/236). Note specific cytoplasmic staining of cystic-epithelial cells (E–G, arrows) but not surrounding normal epithelium (E and F, arrowheads; and I). The cyst-specific cytoplasmic immunosignal is abolished after rapamycin treatment (H, arrows). (Scale bars, 20 μm.)
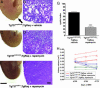
Fig. 3.
Rapamycin treatment significantly improves renal cystogenesis in the _orpk_-rescue mouse model. Day-150 postpartum _orpk_-rescue (Tg737orpk/orpk;TgRsq) mutant mice and _orpk_-heterozygous-rescue (Tg737orpk/+;TgRsq) control mice were given daily i.p. injections of rapamycin (5 mg/kg of body weight) or vehicle for 28 days before isolation, fixation, and sectioning of whole kidneys. (A_–_F) Representative whole kidney (A, C, and E) and H&E-stained sections (B, D, and F) derived from non-rapamycin-treated (A and B) or rapamycin-treated (C and D) (5 mg/kg of body weight) _orpk_-rescue mutant mice and _orpk_-heterozygous-rescue (E and F) control mice. Note the nonuniform appearance of the nontreated mutant kidney (A, arrow) compared with the smooth surface of the treated mutant kidney (C). (G) Cystic indices were calculated based on representative renal sections derived from non-rapamycin-treated and rapamycin-treated _orpk_-rescue mutant mice as described in Materials and Methods. (H) Kidney volumes were measured by MRI scanning of live animals at the beginning (day 150 postpartum), middle (day 164 postpartum), and end (day 178 postpartum) of treatment with rapamycin or vehicle. Each individual line represents a separate kidney for each respective genotype, and the averages for each group are indicated with horizontal lines. [Scale bars, 3 mm (A, C, and E) and 200 μm (B, D, and F).] ∗∗∗, P < 0.001.
Fig. 4.
Rapamycin treatment significantly improves the renal cystic phenotype and kidney function in the bpk mouse model. Mutant (bpk/bpk) and control (bpk/+) mice received daily i.p. injections of rapamycin (5 or 1.67 mg/kg of body weight) starting at day 7 for a period of 14 days. (A_–_C) Representative low-power (Left) and high-power (Right) H&E-stained sections derived from non-rapamycin-treated (A) or rapamycin-treated (B) (5 mg/kg of body weight) bpk/bpk mutant and bpk/+ (C) animals. (D) Kidney weight expressed as a percentage of total body weight. (E) Cystic indices were calculated based on representative renal sections derived from non-rapamycin-treated and rapamycin-treated bpk/bpk mutant mice (n = 4 kidneys) as described in Materials and Methods. (F) Blood drawn from anesthetized animals was assayed for blood urea nitrogen (BUN) according to standard procedures. [Scale bars, 3 mm (A_–_C, whole kidney H&E) and 200 μm (A_–_C, histology H&E).] ∗∗∗, P < 0.001; ∗∗, P < 0.01.
Fig. 5.
Rapamycin treatment induces increased apoptosis and luminal shedding of cystic epithelial cells. _Orpk_-rescue (Tg737orpk/orpk;TgRsq) mutant and _orpk_-heterozygous-rescue (Tg737orpk/+;TgRsq) control mice were treated with rapamycin or vehicle as indicated. Renal tissue sections were processed and analyzed for the presence of apoptotic cells by TUNEL. (A) TUNEL-positive, cyst-lining epithelial cells were observed in sections of nontreated Tg737orpk/orpk;TgRsq mutant mice, whereas a significant increase in TUNEL-positive, cyst-lining and cyst-luminal epithelial cells were observed in sections of rapamycin-treated Tg737orpk/orpk;TgRsq mutant mice. (B) Quantification of TUNEL-positive cells in renal sections derived from vehicle or rapamycin-treated o_rpk_-rescue mutant animals expressed as an average of TUNEL-positive cells per cyst (n = 4 kidneys). ∗∗∗, P < 0.001.
Comment in
- mTOR is out of control in polycystic kidney disease.
Mostov KE. Mostov KE. Proc Natl Acad Sci U S A. 2006 Apr 4;103(14):5247-8. doi: 10.1073/pnas.0601352103. Epub 2006 Mar 27. Proc Natl Acad Sci U S A. 2006. PMID: 16567652 Free PMC article. No abstract available.
Similar articles
- Regulation of mTOR by polycystin-1: is polycystic kidney disease a case of futile repair?
Weimbs T. Weimbs T. Cell Cycle. 2006 Nov 1;5(21):2425-9. doi: 10.4161/cc.5.21.3408. Epub 2006 Sep 13. Cell Cycle. 2006. PMID: 17102641 Review. - Ablation of Long Noncoding RNA Hoxb3os Exacerbates Cystogenesis in Mouse Polycystic Kidney Disease.
Weisser I, Eckberg K, D'Amico S, Buttram D, Aboudehen K. Weisser I, et al. J Am Soc Nephrol. 2024 Jan 1;35(1):41-55. doi: 10.1681/ASN.0000000000000265. Epub 2023 Nov 13. J Am Soc Nephrol. 2024. PMID: 37953472 Free PMC article. - Double inhibition of cAMP and mTOR signalling may potentiate the reduction of cell growth in ADPKD cells.
de Stephanis L, Bonon A, Varani K, Lanza G, Gafà R, Pinton P, Pema M, Somlo S, Boletta A, Aguiari G. de Stephanis L, et al. Clin Exp Nephrol. 2017 Apr;21(2):203-211. doi: 10.1007/s10157-016-1289-1. Epub 2016 Jun 9. Clin Exp Nephrol. 2017. PMID: 27278932 Free PMC article. - An mTOR anti-sense oligonucleotide decreases polycystic kidney disease in mice with a targeted mutation in Pkd2.
Ravichandran K, Zafar I, He Z, Doctor RB, Moldovan R, Mullick AE, Edelstein CL. Ravichandran K, et al. Hum Mol Genet. 2014 Sep 15;23(18):4919-31. doi: 10.1093/hmg/ddu208. Epub 2014 May 8. Hum Mol Genet. 2014. PMID: 24847003 Free PMC article. - Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1.
Weimbs T. Weimbs T. Am J Physiol Renal Physiol. 2007 Nov;293(5):F1423-32. doi: 10.1152/ajprenal.00275.2007. Epub 2007 Aug 22. Am J Physiol Renal Physiol. 2007. PMID: 17715262 Review.
Cited by
- Deletion of Aurora kinase A prevents the development of polycystic kidney disease in mice.
Tham MS, Cottle DL, Zylberberg AK, Short KM, Jones LK, Chan P, Conduit SE, Dyson JM, Mitchell CA, Smyth IM. Tham MS, et al. Nat Commun. 2024 Jan 8;15(1):371. doi: 10.1038/s41467-023-44410-9. Nat Commun. 2024. PMID: 38191531 Free PMC article. - Rapamycin-mediated suppression of renal cyst expansion in del34 Pkd1-/- mutant mouse embryos: an investigation of the feasibility of renal cyst prevention in the foetus.
Stayner C, Shields J, Slobbe L, Shillingford JM, Weimbs T, Eccles MR. Stayner C, et al. Nephrology (Carlton). 2012 Nov;17(8):739-47. doi: 10.1111/j.1440-1797.2012.01639.x. Nephrology (Carlton). 2012. PMID: 22725947 Free PMC article. - Shared pathobiology identifies AMPK as a therapeutic target for obesity and autosomal dominant polycystic kidney disease.
Iliuta IA, Song X, Pickel L, Haghighi A, Retnakaran R, Scholey J, Sung HK, Steinberg GR, Pei Y. Iliuta IA, et al. Front Mol Biosci. 2022 Aug 23;9:962933. doi: 10.3389/fmolb.2022.962933. eCollection 2022. Front Mol Biosci. 2022. PMID: 36106024 Free PMC article. Review. - Renal Ciliopathies: Sorting Out Therapeutic Approaches for Nephronophthisis.
Stokman MF, Saunier S, Benmerah A. Stokman MF, et al. Front Cell Dev Biol. 2021 May 13;9:653138. doi: 10.3389/fcell.2021.653138. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34055783 Free PMC article. Review. - Cyst Reduction in a Polycystic Kidney Disease Drosophila Model Using Smac Mimics.
Millet-Boureima C, Chingle R, Lubell WD, Gamberi C. Millet-Boureima C, et al. Biomedicines. 2019 Oct 18;7(4):82. doi: 10.3390/biomedicines7040082. Biomedicines. 2019. PMID: 31635379 Free PMC article.
References
- Torres V. E., Harris P. C. Nefrologia. 2003;23:14–22. - PubMed
- Igarashi P., Somlo S. J. Am. Soc. Nephrol. 2002;13:2384–2398. - PubMed
- Wilson P. D. N. Engl. J. Med. 2004;350:151–164. - PubMed
- Sutters M., Germino G. G. J. Lab. Clin. Med. 2003;141:91–101. - PubMed
- Ong A. C., Harris P. C. Kidney Int. 2005;67:1234–1247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 DK057306/DK/NIDDK NIH HHS/United States
- R01 DK062338/DK/NIDDK NIH HHS/United States
- P50 DK 57306/DK/NIDDK NIH HHS/United States
- R01 DK 62338/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous