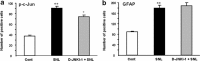A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance - PubMed (original) (raw)
A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance
Zhi-Ye Zhuang et al. J Neurosci. 2006.
Abstract
Optimal management of neuropathic pain is a major clinical challenge. We investigated the involvement of c-Jun N-terminal kinase (JNK) in neuropathic pain produced by spinal nerve ligation (SNL) (L5). SNL induced a slow (>3 d) and persistent (>21 d) activation of JNK, in particular JNK1, in GFAP-expressing astrocytes in the spinal cord. In contrast, p38 mitogen-activated protein kinase activation was found in spinal microglia after SNL, which had fallen to near basal level by 21 d. Intrathecal infusion of a JNK peptide inhibitor, D-JNKI-1, did not affect normal pain responses but potently prevented and reversed SNL-induced mechanical allodynia, a major symptom of neuropathic pain. Intrathecal D-JNKI-1 also suppressed SNL-induced phosphorylation of the JNK substrate, c-Jun, in spinal astrocytes. However, SNL-induced upregulation of GFAP was not attenuated by spinal D-JNKI-1 infusion. Furthermore, SNL induced a rapid (<12 h) but transient activation of JNK in the L5 (injured) but not L4 (intact) DRG. JNK activation in the DRG was mainly found in small-sized C-fiber neurons. Infusion of D-JNKI-1 into the L5 DRG prevented but did not reverse SNL-induced mechanical allodynia. Finally, intrathecal administration of an astroglial toxin, l-alpha-aminoadipate, reversed mechanical allodynia. Our data suggest that JNK activation in the DRG and spinal cord play distinct roles in regulating the development and maintenance of neuropathic pain, respectively, and that spinal astrocytes contribute importantly to the persistence of mechanical allodynia. Targeting the JNK pathway in spinal astroglia may present a new and efficient way to treat neuropathic pain symptoms.
Figures
Figure 1.
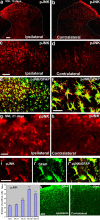
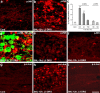
a–l, SNL induces persistent JNK activation in spinal astroglia. a, b, Immunohistochemistry reveals increases in pJNK levels in the ipsilateral spinal dorsal horn (L5) 10 d after SNL. White lines indicate the border of the dorsal horn gray matter. Scale bar, 50 μm. c, d, High-magnification images from a and b, respectively, showing pJNK staining in the medial superficial dorsal horn. Scale bar, 50 μm. e, Double immunofluorescence shows that pJNK (red) is completely colocalized with astroglia marker GFAP (green) in the medial superficial dorsal horn. Two single-stained images were merged. e has the same magnification as c. f, High-magnification image from e demonstrating the colocalization of pJNK and GFAP. Scale bar, 25 μm. g, h, SNL also induces JNK activation in the dorsal horn after 3 weeks. Scale bar, 25 μm. i, Colocalization of pJNK (i) and GFAP (i′) in the superficial dorsal horn 21 d after SNL. i″ is the merge of i and i′. Scale bar, 25 μm. j, Time course of pJNK induction in the L5 spinal cord after SNL, as indicated by the number of pJNK-IR cells in the superficial dorsal horn (laminas I–III). **p < 0.01 by ANOVA compared with control (n = 3 and 4). k, l, SNL induces persistent activation of astrocytes in the dorsal horn after 3 weeks, as indicated by GFAP upregulation. Scale bar, 50 μm.
Figure 2.
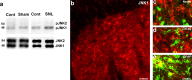
a–e, JNK1 is activated in spinal astrocytes. a, Western blot analysis reveals an increase in the level of pJNK1 (46 kDa) in the L5 spinal cord 10 d after L5 SNL. b, Immunohistochemistry shows JNK1 expression in the medial superficial spinal cord 10 d after SNL. Scale bar, 20 μm. c–e, High-magnification images reveal double staining between JNK1 (red) and NeuN (c), OX-42 (d), or GFAP (e). Two single-stained images were merged. Scale bar, 10 μm. Note that the JNK1 is only colocalized with GFAP.
Figure 3.
a–d, SNL induces phosphorylation of c-Jun in spinal astroglia. a, b, Immunohistochemistry shows increases in p-c-Jun levels in the ipsilateral spinal dorsal horn (L5) 10 d after SNL. White lines indicate the border of the dorsal horn gray matter. Scale bar, 100 μm. c, Double immunofluorescence shows that p-c-Jun (red) is completely colocalized with astroglia marker GFAP (green) in the medial superficial dorsal horn. Scale bar, 50 μm. d, High-magnification image demonstrating the colocalization of pJNK and GFAP. Two single-stained images were merged. Note that p-c-Jun is expressed in the nucleus of GFAP-labeled cells. Scale bar, 10 μm.
Figure 4.
a–j, SNL activates p38 MAPK in spinal microglia. a–f, p-p38 immunofluorescence in the dorsal horn of the ipsilateral (Ipsi; a, c, d, f) and contralateral (Contra; b, e) side at 3 d (a–c) and 21 d (d–f) after SNL. c and d are double staining for p-p38 (red) and OX-42 (green). Note that p-p38 is expressed in OX-42-IR microglia at 3 and 21 d. Two single-stained images were merged. Scale bars: b, e, 100 μm; c, f, 50 μm. The asterisk indicates an artifact. g–j, OX-42 immunofluorescence in the dorsal horn of the ipsilateral (g, i) and contralateral (h, j) side at 3 d (g, h) and 21 d (i, j) after SNL. Scale bar, 100 μm.
Figure 5.
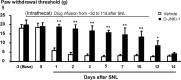
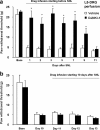
Intrathecal perfusion of D-JNKI-1 prevents the development of neuropathic pain. The peptide JNK inhibitor D-JNKI-1 (50 μ
m
) was infused into intrathecal space via an osmotic pump (0.5 μl/h for 2 weeks), starting 3 d before SNL. Note that the basal mechanical sensitivity is not changed after D-JNKI-1 infusion. The inhibitor almost completely blocks allodynia for >10 d, but neuropathic pain returns after infusion stops. *p < 0.05, **p < 0.01 by t test compared with corresponding vehicle controls (saline); n = 6.
Figure 6.
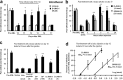
a–d, Intrathecal administration of JNK inhibitors reverses neuropathic pain. a, Reversal of SNL-induced mechanical allodynia by intrathecal infusion of D-JNKI-1 (50 μ
m
) via an osmotic pump (0.5 μl/h for 3 d) starting on post-SNL day 10. *p < 0.05 by t test compared with corresponding saline controls; n = 5. b, Reversal of SNL-induced mechanical allodynia by a bolus intrathecal injection of D-JNKI-1 (4 nmol) and SP600125 (50 nmol) at post-SNL day 10. *p < 0.05, **p < 0.01 by ANOVA compared with corresponding preinjection baseline; n = 6. c, Effects of D-JNKI-1 (0.2 and 4 nmol) and its control peptide (4 nmol), SP600125 (SP; 50 and 150 nmol) and its vehicle (20% DMSO), and saline, via intrathecal bolus injection, on mechanical allodynia on post-SNL day 10. **p < 0.01 compared with the vehicle (Veh); ##p < 0.01, compared with the control peptide; n = 6. The mechanical allodynia was tested at 3 h after all of the injections. d, Dose-dependent effects of D-JNKI-1 and SP600125, intrathecally injected on day 10, on reversing mechanical allodynia. Mechanical allodynia was tested 3 h after the injections. The ED50 for D-JNKI-1 and SP600125 is 0.3 and 14.9 nmol, respectively (n = 6).
Figure 7.
a, b, Effects of intrathecal D-JNKI-1 on SNL-induced c-Jun phosphorylation and GFAP expression in astrocytes. The numbers of p-c-Jun-IR (a) and GFAP-IR (b) cells in the medial two-thirds of superficial dorsal horn (laminas I–III) at 10 d after SNL were quantified. D-JNKI-1 was intrathecally infused via an osmotic pump (0.5 μl/h, 50 μ
m
) at 7 d after SNL, and animals were perfused 3 d after the infusion. **p < 0.01, compared with controls; +p < 0.05, compared with SNL, ANOVA; n = 4.
Figure 8.
Uptake of the Tat peptide by DRG cells after intrathecal injection. Many fluorescence-labeled neurons and satellite cells are present in the DRG 3 h after intrathecal injection of a cumarin-labeled Tat peptide (4 nmol). Large and small arrows indicate neurons and satellite cells, respectively. Scale bar, 50 μm.
Figure 9.
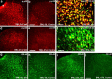
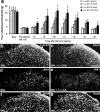
a–i, SNL induces rapid but transient JNK activation in the injured (L5) neurons of the DRG. a, b, SNL produces a rapid (at 12 h) pJNK increase in small-sized neurons of the L5 DRG. c, Percentage of pJNK-positive neuronal profiles in the L5 and L4 DRGs at different times after SNL. **p < 0.01 by ANOVA compared with control (naive); n = 4. d, Double immunofluorescence of pJNK (red) and NF-200 (green) in the L5 DRG. Note that pJNK is barely colocalized with NF-200. e, f, There is only low level of pJNK expression in the L5 DRG on day 10 (e) and in the L4 DRG on day 0.5 (f) after SNL. g–i, SNL induces p-c-Jun in the L5 DRG (h) but not in the L4 DRG (i). Scale bar, 50 μm.
Figure 10.
a, b, Perfusion of the L5 DRG with D-JNKI-1 prevents but does not reverse mechanical allodynia after SNL. The peptide JNK inhibitor D-JNKI-1 (50 μ
m
) was infused into the L5 DRG via an osmotic pump (0.5 μl/h), starting 30 min before (a) or 10 d after (b) SNL. *p < 0.05 by t test compared with corresponding vehicle controls (saline); n = 5.
Figure 11.
a–g, Astroglial toxin
l
-α-AA blocks neuropathic pain. a,
l
-α-AA (10, 50, and 150 nmol) was injected intrathecally at 10 d after SNL and reversed mechanical allodynia. *p < 0.05, **p < 0.01 by ANOVA compared with vehicle (saline) control; n = 6. b–g, Effects of
l
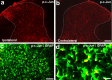
-α-AA (50 nmol) on GFAP (b, c), JNK1 (d, e), and NeuN (f, g) expressing cells in the superficial dorsal horn. Animals with SNL were perfused 24 h after the toxin injection for immunostaining. Scale bar, 50 μm.
Similar articles
- Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation.
Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Obata K, et al. J Neurosci. 2004 Nov 10;24(45):10211-22. doi: 10.1523/JNEUROSCI.3388-04.2004. J Neurosci. 2004. PMID: 15537893 Free PMC article. - Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons.
Schäfers M, Svensson CI, Sommer C, Sorkin LS. Schäfers M, et al. J Neurosci. 2003 Apr 1;23(7):2517-21. doi: 10.1523/JNEUROSCI.23-07-02517.2003. J Neurosci. 2003. PMID: 12684435 Free PMC article. - Inhibition of spinal astrocytic c-Jun N-terminal kinase (JNK) activation correlates with the analgesic effects of ketamine in neuropathic pain.
Mei XP, Zhang H, Wang W, Wei YY, Zhai MZ, Wang W, Xu LX, Li YQ. Mei XP, et al. J Neuroinflammation. 2011 Jan 24;8(1):6. doi: 10.1186/1742-2094-8-6. J Neuroinflammation. 2011. PMID: 21255465 Free PMC article. - Activation of JNK pathway in persistent pain.
Gao YJ, Ji RR. Gao YJ, et al. Neurosci Lett. 2008 Jun 6;437(3):180-3. doi: 10.1016/j.neulet.2008.03.017. Epub 2008 Mar 13. Neurosci Lett. 2008. PMID: 18455869 Free PMC article. Review. - Goshajinkigan attenuates paclitaxel-induced neuropathic pain via cortical astrocytes.
Takanashi K, Shibata K, Mizuno K, Komatsu R, Koizumi S. Takanashi K, et al. Pharmacol Res Perspect. 2021 Dec;9(6):e00850. doi: 10.1002/prp2.850. Pharmacol Res Perspect. 2021. PMID: 34676996 Free PMC article. Review.
Cited by
- Development of opioid-induced hyperalgesia depends on reactive astrocytes controlled by Wnt5a signaling.
Liu X, Bae C, Liu B, Zhang YM, Zhou X, Zhang D, Zhou C, DiBua A, Schutz L, Kaczocha M, Puopolo M, Yamaguchi TP, Chung JM, Tang SJ. Liu X, et al. Mol Psychiatry. 2023 Feb;28(2):767-779. doi: 10.1038/s41380-022-01815-0. Epub 2022 Oct 6. Mol Psychiatry. 2023. PMID: 36203006 Free PMC article. - Comparison of central versus peripheral delivery of pregabalin in neuropathic pain states.
Martinez JA, Kasamatsu M, Rosales-Hernandez A, Hanson LR, Frey WH, Toth CC. Martinez JA, et al. Mol Pain. 2012 Jan 11;8:3. doi: 10.1186/1744-8069-8-3. Mol Pain. 2012. PMID: 22236461 Free PMC article. Retracted. - Inhibitors of c-Jun N-terminal kinases: JuNK no more?
Bogoyevitch MA, Arthur PG. Bogoyevitch MA, et al. Biochim Biophys Acta. 2008 Jan;1784(1):76-93. doi: 10.1016/j.bbapap.2007.09.013. Epub 2007 Oct 11. Biochim Biophys Acta. 2008. PMID: 17964301 Free PMC article. Review. - Nefopam downregulates autophagy and c-Jun N-terminal kinase activity in the regulation of neuropathic pain development following spinal nerve ligation.
Oh SH, Yoon MH, Lim KJ, Yu BS, Jee IG, Jung KT. Oh SH, et al. BMC Anesthesiol. 2018 Jul 27;18(1):97. doi: 10.1186/s12871-018-0559-8. BMC Anesthesiol. 2018. PMID: 30053799 Free PMC article. - A critical role of SRC-suppressed C kinase substrate in rat astrocytes after chronic constriction injury.
Xia Y, Liu H, Shen A, Liu Y, Sun L, Tao T, Ke Q, Cheng C. Xia Y, et al. Neuromolecular Med. 2010 Sep;12(3):205-16. doi: 10.1007/s12017-009-8093-y. Epub 2009 Nov 25. Neuromolecular Med. 2010. PMID: 19937403
References
- Borsello T, Bonny C (2004). Use of cell-permeable peptides to prevent neuronal degeneration. Trends Mol Med 10:239–244. - PubMed
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C (2003). A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 9:1180–1186. - PubMed
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. - PubMed
- Coggeshall RE, Lekan HA (1996). Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol 364:6–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous