A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis - PubMed (original) (raw)
A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis
Zhong Zhao et al. BMC Neurosci. 2006.
Abstract
Background: The cause of neuronal death in amyotrophic lateral sclerosis (ALS) is uncertain but mitochondrial dysfunction may play an important role. Ketones promote mitochondrial energy production and membrane stabilization.
Results: SOD1-G93A transgenic ALS mice were fed a ketogenic diet (KD) based on known formulations for humans. Motor performance, longevity, and motor neuron counts were measured in treated and disease controls. Because mitochondrial dysfunction plays a central role in neuronal cell death in ALS, we also studied the effect that the principal ketone body, D-beta-3 hydroxybutyrate (DBH), has on mitochondrial ATP generation and neuroprotection. Blood ketones were > 3.5 times higher in KD fed animals compared to controls. KD fed mice lost 50% of baseline motor performance 25 days later than disease controls. KD animals weighed 4.6 g more than disease control animals at study endpoint; the interaction between diet and change in weight was significant (p = 0.047). In spinal cord sections obtained at the study endpoint, there were more motor neurons in KD fed animals (p = 0.030). DBH prevented rotenone mediated inhibition of mitochondrial complex I but not malonate inhibition of complex II. Rotenone neurotoxicity in SMI-32 immunopositive motor neurons was also inhibited by DBH.
Conclusion: This is the first study showing that diet, specifically a KD, alters the progression of the clinical and biological manifestations of the G93A SOD1 transgenic mouse model of ALS. These effects may be due to the ability of ketone bodies to promote ATP synthesis and bypass inhibition of complex I in the mitochondrial respiratory chain.
Figures
Figure 1
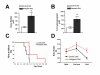
Ketone levels, body weight motor function. (A) The serum content of total ketone bodies (DHB and acetoacetate) in SOD1-G93A transgenic mice. (data = mean ± se; standard diet: n = 5, KD: n = 6; * - p < 0.05). (B) The serum content of DHB in SOD1-G93A transgenic mice. (data = mean ± se; standard diet: n = 5, KD: n = 6; * - p < 0.05). (C) Time to failure (50% baseline performance) in SOD1-G93A transgenic mice fed a KD compared to standard diet from 85 days to 136 days of age. (data = mean ± se; standard diet: n = 5, KD: n = 6; p = 0.027). (D) The effect of the KD on weight in SOD1-G93A mice at the beginning of treatment (Day 50), presymptomatic (Day 89) and endpoint of the study. (data = mean ± se; standard diet: n = 5, KD: n = 6[AV3]; p = 0.047).
Figure 2
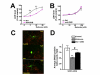
Nissl-stained motor neuron count in the lumbar spinal cord. The effect of the KD on Nissl-stained neuronal cell counts in SOD1-G93A transgenic mice at study endpoint. (A) Photomicrographs of representative Nissl-stained sections through the ventral horns of the lumbar spinal cord from wild-type (WT) (a), standard diet- (b) and KD (c) treated SOD1-G93A mice. Horizontal bar = 25 μm. (B) The counts of the large motor neurons in lumbar spinal cord of WT control and of SOD1-G93A treated with standard diet or KD (data = mean ± se; WT: n = 5, Tg G93A: n = 4, Tg G93A+KD n = 6; ## - p = 0.003, Tg G93A vs. Tg G93A+KD; *** - p < 0.001 vs. WT).
Figure 3
Differential effect of DHB on mitochondrial activity. (A) Representative examples of ATP synthesis measurements in spinal cord expressed as relative luminescence units (R.L.U.). (B) The rate of ATP synthesis in SOD1-G93A mitochondria purified from spinal cord (data = mean ± se; repeated measures, n = 9; ** - p < 0.001). (C) Effect of DHB on rotenone mediated cell viability. Primary mixed spinal cord neurons from SOD1-G93A mice were cultured in the presence or absence of DHB (5 mM) and exposed to mitochondrial Complex I inhibitor rotenone (data = mean ± se; Rot: n = 4, Rot+DHB: n = 4; *** - p < 0.001, Rot vs. Rot+DHB). (D) Absence of specific inhibition of DHB in SOD1-G93A cultures exposed to mitochondrial Complex II inhibitor malonate (data = mean ± se; Mal: n = 4, Mal+DHB: n = 4).
Figure 4
Neuroprotective effects of DBH on spinal cord motor neurons. (A) Effect of DHB on rotenone mediated toxicity. Primary spinal cord neurons from SOD1-G93A mice were cultured in the presence or absence of DHB (5 mM) and exposed to rotenone and cell toxicity measured by LDH activity released in the culture media expressed as a percentage of control (data = mean ± se; Rot: n = 4, Rot+DHB: n = 4; ** - p < 0.01, Rot vs. Rot+DHB). (B) Absence of neuroprotection of DHB in SOD1-G93A cultures exposed to malonate expressed as a percentage of control (data = mean ± se; Mal: n = 4, Mal+DHB: n = 4). (C) Photomicrographs of representative staining of motor neurons using SMI-32 antibody in control (top), rotenone (middle) and rotenone and DHB (bottom) treated spinal cord cultures. Motor neurons are SMI32 positive and characterized by their large cell bodies and numerous dendrites. (D) The effect of DHB on chronic mitochondrial toxicity by rotenone. Spinal cords neurons from SOD1-G93A were cultured in the presence or absence of DHB (5 mM) and treated with rotenone (3 nM) (data = mean ± se; Control: n = 4, Rotenone: n = 4, Rot+DHB: n = 4; # - p = 0.041, Rotenone vs. Rot+DHB; ** - p = 0.004, Rotenone vs. Control).
Figure 5
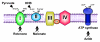
Scheme 1 : Mitochondrial enzyme complexes of the respiratory chain and inhibition sites.
Similar articles
- Modulation of astrocytic mitochondrial function by dichloroacetate improves survival and motor performance in inherited amyotrophic lateral sclerosis.
Miquel E, Cassina A, Martínez-Palma L, Bolatto C, Trías E, Gandelman M, Radi R, Barbeito L, Cassina P. Miquel E, et al. PLoS One. 2012;7(4):e34776. doi: 10.1371/journal.pone.0034776. Epub 2012 Apr 3. PLoS One. 2012. PMID: 22509356 Free PMC article. - Knocking down metabotropic glutamate receptor 1 improves survival and disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.
Milanese M, Giribaldi F, Melone M, Bonifacino T, Musante I, Carminati E, Rossi PI, Vergani L, Voci A, Conti F, Puliti A, Bonanno G. Milanese M, et al. Neurobiol Dis. 2014 Apr;64:48-59. doi: 10.1016/j.nbd.2013.11.006. Epub 2013 Dec 19. Neurobiol Dis. 2014. PMID: 24361555 - Toll-Like Receptor-4 Inhibitor TAK-242 Attenuates Motor Dysfunction and Spinal Cord Pathology in an Amyotrophic Lateral Sclerosis Mouse Model.
Fellner A, Barhum Y, Angel A, Perets N, Steiner I, Offen D, Lev N. Fellner A, et al. Int J Mol Sci. 2017 Aug 1;18(8):1666. doi: 10.3390/ijms18081666. Int J Mol Sci. 2017. PMID: 28763002 Free PMC article. - How Can a Ketogenic Diet Improve Motor Function?
Veyrat-Durebex C, Reynier P, Procaccio V, Hergesheimer R, Corcia P, Andres CR, Blasco H. Veyrat-Durebex C, et al. Front Mol Neurosci. 2018 Jan 26;11:15. doi: 10.3389/fnmol.2018.00015. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29434537 Free PMC article. Review. - Therapeutic alternative of the ketogenic Mediterranean diet to improve mitochondrial activity in Amyotrophic Lateral Sclerosis (ALS): A Comprehensive Review.
Caplliure-Llopis J, Peralta-Chamba T, Carrera-Juliá S, Cuerda-Ballester M, Drehmer-Rieger E, López-Rodriguez MM, de la Rubia Ortí JE. Caplliure-Llopis J, et al. Food Sci Nutr. 2019 Dec 16;8(1):23-35. doi: 10.1002/fsn3.1324. eCollection 2020 Jan. Food Sci Nutr. 2019. PMID: 31993129 Free PMC article. Review.
Cited by
- AMPK Signalling and Defective Energy Metabolism in Amyotrophic Lateral Sclerosis.
Perera ND, Turner BJ. Perera ND, et al. Neurochem Res. 2016 Mar;41(3):544-53. doi: 10.1007/s11064-015-1665-3. Epub 2015 Jul 23. Neurochem Res. 2016. PMID: 26202426 Review. - Novel mitochondrial targets for neuroprotection.
Perez-Pinzon MA, Stetler RA, Fiskum G. Perez-Pinzon MA, et al. J Cereb Blood Flow Metab. 2012 Jul;32(7):1362-76. doi: 10.1038/jcbfm.2012.32. Epub 2012 Mar 28. J Cereb Blood Flow Metab. 2012. PMID: 22453628 Free PMC article. Review. - Antioxidants prevent inflammation and preserve the optic projection and visual function in experimental neurotrauma.
Bernardo-Colón A, Vest V, Clark A, Cooper ML, Calkins DJ, Harrison FE, Rex TS. Bernardo-Colón A, et al. Cell Death Dis. 2018 Oct 26;9(11):1097. doi: 10.1038/s41419-018-1061-4. Cell Death Dis. 2018. PMID: 30367086 Free PMC article. - Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity.
Yang X, Cheng B. Yang X, et al. J Mol Neurosci. 2010 Oct;42(2):145-53. doi: 10.1007/s12031-010-9336-y. Epub 2010 Mar 24. J Mol Neurosci. 2010. PMID: 20333481 - Anti-Neuroinflammatory Effect of Jaeumganghwa-Tang in an Animal Model of Amyotrophic Lateral Sclerosis.
Lee SH, Yang EJ. Lee SH, et al. Evid Based Complement Alternat Med. 2019 Feb 12;2019:1893526. doi: 10.1155/2019/1893526. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 30891075 Free PMC article.
References
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl. 2000;59:133–154. - PubMed
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, Oregan JP, Deng HX, Rahmani Z, Krizus A, Mckennayasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Vandenbergh R, Hung WY, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericakvance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH. Mutations in Cu/Zn Superoxide-Dismutase Gene Are Associated with Familial Amyotrophic-Lateral-Sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous