Detailed genomic analysis of the Wbeta and gamma phages infecting Bacillus anthracis: implications for evolution of environmental fitness and antibiotic resistance - PubMed (original) (raw)
Comparative Study
Detailed genomic analysis of the Wbeta and gamma phages infecting Bacillus anthracis: implications for evolution of environmental fitness and antibiotic resistance
Raymond Schuch et al. J Bacteriol. 2006 Apr.
Abstract
Phage-mediated lysis has been an essential laboratory tool for rapidly identifying Bacillus anthracis for more than 40 years, relying on the gamma phage derivative of a Bacillus cereus prophage called W. The complete genomic sequences of the temperate W phage, referred to as Wbeta, and its lytic variant gamma were determined and found to encode 53 open reading frames each, spanning 40,864 bp and 37,373 bp, respectively. Direct comparison of the genomes showed that gamma evolved through mutations at key loci controlling host recognition, lysogenic growth, and possibly host phenotypic modification. Included are a cluster of point mutations at the gp14 tail fiber locus of gamma, encoding a protein that, when fused to green fluorescent protein, binds specifically to B. anthracis. A large 2,003-bp deletion was also identified at the gamma lysogeny module, explaining its shift from a temperate to a lytic lifestyle. Finally, evidence of recombination was observed at a dicistronic Wbeta locus, encoding putative bacterial cell surface-modifying proteins, replaced in gamma with a locus, likely obtained from a B. anthracis prophage, encoding demonstrable fosfomycin resistance. Reverse transcriptase PCR analysis confirmed strong induction at the dicistronic Wbeta locus and at four other phage loci in B. anthracis and/or B. cereus lysogens. In all, this study represents the first genomic and functional description of two historically important phages and is part of a broader investigation into contributions of phage to the B. anthracis life cycle. Initial findings suggest that lysogeny of B. anthracis promotes ecological adaptation, rather than virulence, as with other gram-positive pathogens.
Figures
FIG. 1.
B. anthracis phage morphologies and isolation. (A and B) Transmission electron micrographs of γ phage virions showing its isometric head and long noncontractile tail. (C and D) Single colonies of B. cereus ATCC 11950 grown for 16 h on BHI agar plates with and without 20 μg ml−1 fosfomycin, respectively. The central holes in panel C are enriched for the Wβ phage. (E) A single intact Wβ phage virion. All scale bars represent 50 nm.
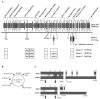
FIG. 2.
Genometric analysis of the γ and Wβ phages. (A) Schematic representation of the entire Wβ phage genome. Numerical gene designations, directions of transcription, and assumed functions are indicated. ORFs 24 and 25 overlap in opposite orientations. The sites of five inserts, deletions, and point mutations that distinguish the γd'Herelle phage (referred to as γ) are shown below the solid line with γ gene number designations. Below the γ phage representation, genetic differences that define the other γ phage isolates, γL, γU, and γC and Cherry, are indicated in the boxed areas below the appropriate genetic positions. The presence or absence of alterations identical to those in d'Herelle is indicated by a plus or a minus sign below gp2, the 28-bp deletion between gp25 and gp26, and the Fosr island (gp39 to gp41). The 2,003-bp deletion in the γ lysogeny region (forming gp28) is observed in each isolate, although the 3′ end extends an additional 640 bp in the cases of isolates γL and γC. A set of only four point mutations at orf14 (identical in isolates γL, γU, and γC and distinct from d'Herelle) is shown. The orf15 locus, identical in Wβ and d'Herelle, is distinguished by a set of 818 base differences, identical in isolates γL, γU and γC. Other than the mutations described here, the Wβ sequence is 100% identical to that of the γL, γU and γC, and the d'Herelle phages. (B) Genomic loci of the B. anthracis Ames strain that bear similarity to γ and Wβ phage sequences. GenBank sequences BA3767 to BA3819, BA4066 to BA4126, BA5339 to BA5363, and BA0427 to BA0486 correspond to known Ames prophages (LambdaBa01 to LambdaBa04, respectively). The asterisk denotes five loci with products >40% identical to five contiguous γ and Wβ loci. (C) Pairwise comparison of Wβ loci with homologous sequences of B. anthracis prophages φ4066 and φ3767. Wβ gene numbers are indicated at the top, while prophage gene designations are indicated below (in parentheses) in numerical order. The prophages are represented not as their linear integrated forms but rather as their inverted left and right halves (gene numbers are indicated below in parentheses) in order to maintain gene order with Wβ. Light stippling represents genes encoding proteins with 18 to 30% identity, medium is 31 to 40%, heavy is 41 to 75%, and filled is 76 to 100%. Since Wβ encodes fewer genes, some φ4066 and φ3767 loci are inserted either as small rectangles within the gene order or as rectangles extending below each sequence. For φ3767, the following genes are absent from the GenBank sequence and are not included in the gene order shown: BA3772, BA3773, BA3790, BA3794, and BA3798.
FIG. 3.
RT-PCR analysis of Wβ phage gene expression during the lysogenic state. RNA prepared from cultures grown under three different conditions was reverse transcribed with random primers. Subsequent PCR was performed with primers specific for the indicated Wβ phage genes. (A) Analysis with RNA from overnight BHI broth cultures of two different B. anthracis (ΔSterne::φWβ) lysogens (+) and the parental nonlysogen (−) and from the B. cereus (RSVF1::φWβ) lysogen (+) and nonlysogen (−). (B) Analysis of RNA isolated from only B. anthracis and B. cereus Wβ lysogens grown for 5 h in either liquid sporulation medium (+) or BHI medium (−).
FIG. 4.
Tail fiber sequence and binding analysis. (A) Sequence alignment of the Gp14 and Wp14 tail fibers of γ and Wβ, respectively, with Sp14 (a plaque size variant of Wβ), Cp14 (of the B. anthracis Cherry phage, accession number YP_338146), and BA4079 (of the B. anthracis φ4066 prophage, accession number NP_846318). Conserved residues are indicated by dark boxes, while conservative changes are indicated by gray boxes. (B) Fluorescence micrograph of GFP-Gp14 binding to the surface of B. anthracis ΔSterne. (C) GFP-Gp14 binding to the surface of B. cereus RSVF1. The contrast in this image was manipulated with Adobe Photoshop 7.0 in order to better illustrate binding. The actual polar/septal fluorescence signal was more akin to that observed in the background of panel D. (D) Rare whole-cell binding of GFP-Gp14 binding to B. cereus RSVF1. (E) Phase-contrast image of B. cereus ATCC 10987 after treatment with GFP-Gp14. (F) Fluorescence image corresponding to the field shown in panel E. (G) Fluorescence-based method for distinguishing B. anthracis from B. cereus and B. thuringiensis in pure and mixed samples. The indicated bacterial organisms were treated with the GFP-Gp14 fusion, washed, and then examined to quantify retained surface fluorescence. The results represent averages of three independent experiments. Bc, B. cereus; Ba, B. anthracis; Bt, B. thuringiensis.
FIG. 5.
Acquisition of the gp41 locus and the role for its product in fosfomycin resistance. (A) Pairwise comparison of the _gp39_-to-gp41 γ phage locus to the corresponding BA4111_-to-BA4009 region of the B. anthracis φ4066 prophage. Bold values between aligned phage sequences indicate the percent identity of the respective protein products. The 5′ 21-bp and 3′ 230-bp regions of DNA sequence homology (100% and 88.4% identity, respectively) shared by φ4066 and γ (and Wβ as well), and presumed to support recombination, are indicated as the cross-hatched regions extending above the γ phage sequence. The cross-hatching between genomes indicates the region of 100% DNA identity observed between γ and φ4066 but absent in Wβ. Arrows indicate the direction of gene transcription. Flanking loci are not homologous. (B) Phylogenetic representation of sequence relationships between fosfomycin resistance proteins from a variety of bacterial species. Most are encoded in the host chromosome, with the exception of E. coli Fosr (transposon Tn_2921 of plasmid pSU961), B. anthracis BA4109 (φ4066 encoded), Staphylococcus epidermidis (plasmid pIP1842), and Pseudomonas aeruginosa (class I integron). The consensus tree was obtained by a bootstrap analysis of an alignment (1,000 replicates) obtained with the MacVector program (Accelrys Inc.). Nodes that are well supported by the phylogenetic analysis are indicated by the percentages of replicates for which those nodes were recovered. The root was arbitrarily set. (C) Fosfomycin resistance conferred by Gp41. Bacterial cultures were plated in the presence of increasing concentrations of fosfomycin, and resultant colonies were enumerated. RSVF1 (open squares), RSVF1/pDG148 (cross-hatches), and RSVF1/pDG148::gp41 (open circles) are shown. (D) Inherent fosfomycin resistance in B. anthracis and other soil organisms. The following strains were plated in the presence of increasing concentrations of fosfomycin, and the resulting colonies were enumerated: B. cereus RSVF1 (open squares), B. cereus ATCC 14579 (closed circles), B. cereus ATCC 13472 (closed squares), B. thuringiensis HD1 (open triangles), and B. anthracis ΔSterne (closed diamonds). Values represent the averages of four independent experiments.
FIG. 6.
Alignments of the wp40 and wp41 products of Wβ with homologous protein sequences. (A) Alignment of the putative spore surface antigen Wp40 with the collagen triple-helix repeat proteins of B. cereus strain ATCC 14579 (BA4769; accession number NP_834473) and B. cereus strain ATCC10987 (BCE4869; accession number NP_981162). (B) Alignment of the putative mannose 6-phosphate isomerase Wp41 with similar proteins from a variety of bacterial organisms, including Clostridium tetani E88 (CTC01899; accession number NP_782477), B. thuringiensis ATCC 35646 (RBTH_04512; accession number ZP_00741184), B. subtilis 168 (BSU26560; accession number NP_390533), Lactobacillus plantarum (pLP9000_05; accession number NP_631996), and Methanosarcina barkeri (MbarDRAFT_2481; accession number ZP_00543223). Black-shaded residues are identical in the alignment, while gray-shaded residues denote conservative changes.
FIG. 7.
Graded relatedness that defines the Wβ-and-γ group of phages. The flow chart is based on genetic differences described in this work and is intended only to illustrate the mutations and recombination events that currently distinguish each phage. Other than those differences reported here, all phage sequences are identical. The series of alterations is presented in an arbitrary manner and is not intended to imply any true phylogeny. The γ variants listed are γd'H (isolate d'Herelle described in this study), γU (isolate USAMRIID), γL (isolate LSU), and γC (Cherry isolate). aa, amino acids.
Similar articles
- Genome sequence of Bacillus anthracis typing phage AP631.
Liu X, Wang D, Pan C, Feng E, Fan H, Li M, Zhu L, Tong Y, Wang H. Liu X, et al. Arch Virol. 2019 Mar;164(3):917-921. doi: 10.1007/s00705-018-04135-3. Epub 2019 Jan 21. Arch Virol. 2019. PMID: 30666457 Free PMC article. - Genome characteristics of a novel phage from Bacillus thuringiensis showing high similarity with phage from Bacillus cereus.
Yuan Y, Gao M, Wu D, Liu P, Wu Y. Yuan Y, et al. PLoS One. 2012;7(5):e37557. doi: 10.1371/journal.pone.0037557. Epub 2012 May 23. PLoS One. 2012. PMID: 22649540 Free PMC article. - Genomic characterization of three novel Basilisk-like phages infecting Bacillus anthracis.
Farlow J, Bolkvadze D, Leshkasheli L, Kusradze I, Kotorashvili A, Kotaria N, Balarjishvili N, Kvachadze L, Nikolich M, Kutateladze M. Farlow J, et al. BMC Genomics. 2018 Sep 18;19(1):685. doi: 10.1186/s12864-018-5056-4. BMC Genomics. 2018. PMID: 30227847 Free PMC article. - Phages preying on Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: past, present and future.
Gillis A, Mahillon J. Gillis A, et al. Viruses. 2014 Jul 9;6(7):2623-72. doi: 10.3390/v6072623. Viruses. 2014. PMID: 25010767 Free PMC article. Review. - Characterization and comparative genomic analysis of bacteriophages infecting members of the Bacillus cereus group.
Lee JH, Shin H, Ryu S. Lee JH, et al. Arch Virol. 2014 May;159(5):871-84. doi: 10.1007/s00705-013-1920-3. Epub 2013 Nov 22. Arch Virol. 2014. PMID: 24264384 Review.
Cited by
- Identification of Prophages within the Mycobacterium avium 104 Genome and the Link of Their Function Regarding to Environment Survival.
Zhao M, Gilbert K, Danelishvili L, Jeffrey B, Bermudez LE. Zhao M, et al. Adv Microbiol. 2016 Nov;6(13):927-941. doi: 10.4236/aim.2016.613087. Epub 2016 Nov 9. Adv Microbiol. 2016. PMID: 34295573 Free PMC article. - Bacteriophage PBC1 and its endolysin as an antimicrobial agent against Bacillus cereus.
Kong M, Ryu S. Kong M, et al. Appl Environ Microbiol. 2015 Apr;81(7):2274-83. doi: 10.1128/AEM.03485-14. Epub 2015 Jan 16. Appl Environ Microbiol. 2015. PMID: 25595773 Free PMC article. - Subcellular organization of viral particles during maturation of nucleus-forming jumbo phage.
Chaikeeratisak V, Khanna K, Nguyen KT, Egan ME, Enustun E, Armbruster E, Lee J, Pogliano K, Villa E, Pogliano J. Chaikeeratisak V, et al. Sci Adv. 2022 May 6;8(18):eabj9670. doi: 10.1126/sciadv.abj9670. Epub 2022 May 4. Sci Adv. 2022. PMID: 35507660 Free PMC article. - Isolation, Partial Characterization and Application of Bacteriophages in Eradicating Biofilm Formation by Bacillus cereus on Stainless Steel Surfaces in Food Processing Facilities.
Gdoura-Ben Amor M, Culot A, Techer C, AlReshidi M, Adnan M, Jan S, Baron F, Grosset N, Snoussi M, Gdoura R, Gautier M. Gdoura-Ben Amor M, et al. Pathogens. 2022 Aug 2;11(8):872. doi: 10.3390/pathogens11080872. Pathogens. 2022. PMID: 36014993 Free PMC article. - Identification and characterization of two Bacillus anthracis bacteriophages.
Li L, Zhang H, Jin H, Guo J, Liu P, Yang J, Wang Z, Zhang E, Yu B, Shi L, He J, Wang P, Wei J, Zhong Y, Li W. Li L, et al. Arch Virol. 2024 Jun 5;169(7):134. doi: 10.1007/s00705-024-06005-7. Arch Virol. 2024. PMID: 38834736 Free PMC article.
References
- Ackermann, H. W., R. R. Azizbekyan, H. P. Emadi Konjin, M. M. Lecadet, L. Seldin, and M. X. Yu. 1994. New Bacillus bacteriophage species. Arch. Virol. 135:333-344. - PubMed
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
- Armstrong, R. N. 2000. Mechanistic diversity in a metalloenzyme superfamily. Biochemistry 39:13625-13632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical