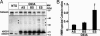Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model - PubMed (original) (raw)
Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model
Hitoshi Kikuchi et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Mutation in superoxide dismutase-1 (SOD1), which is a cause of ALS, alters the folding patterns of this protein. Accumulation of misfolded mutant SOD1 might activate endoplasmic reticulum (ER) stress pathways. Here we show that transgenic mice expressing ALS-linked SOD1 mutants exhibit molecular alterations indicative of a recruitment of ER's signaling machinery. We demonstrate by biochemical and morphological methods that mutant SOD1 accumulates inside the ER, where it forms insoluble high molecular weight species and interacts with the ER chaperone immunoglobulin-binding protein. These alterations are age- and region-specific, because they develop over the course of the disease and occur in the affected spinal cord but not in the nonaffected cerebellum in transgenic mutant SOD1 mice. Our results suggest a toxic mechanism for mutant SOD1 by which this ubiquitously expressed pathogenic protein could affect motor neuron survival and contribute to the selective motor neuronal degeneration in ALS.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
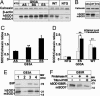
Fig. 1.
Activation of UPR transcription factors and apoptosis-associated ER factors in transgenic SOD1G93A mice. (A) ATF6 immunoblot in spinal cords from transgenic SOD1G93A mice (G93A) at asymptomatic stage (AS, 1 month old), beginning of symptoms (BS, 3–4 months old), end stage (ES, 5 months old), and from their age-matched nontransgenic littermates (NTG) or from the cerebellum (Cb) of ES-G93A mice. Thapsigargin-treated PC12-cells were used as a positive control (Cont.). Full-length ATF6 is p90, and cleaved ATF6 is p50. (B) Immunolocalization of ATF6 in spinal cord sections: ATF6 (green), neurofilament (red), and DAPI (blue). (Scale bar, 20 μm.) (C) Ratios of IRE1-mediated splicing form of XBP1 (spliced -XBP1) over total XBP1 mRNA. ∗, P < 0.05; Student's t test. (D) Immunoblot for phosphorylated IRE1α (_p_-IRE1α) and nonphosphorylated IRE1α (IRE1α). (E) ATF4 immunoblot. ∗, P < 0.05; one-way ANOVA, Student–Newman–Keuls test. (F) Immunoblot of procaspase-12 (≈55 kDa) and its cleaved fragment (≈42 kDa). All values are means ± SEM (n = three to six per group). Analyses were performed in ES-G93A and age-matched NTG mouse spinal cords unless indicated otherwise.
Fig. 2.
SOD1WT and mutant SOD1 are present in the ER. (A) Immunoblot of spinal microsomal fractions of SOD1G93A mice at different disease stages and 5-month-old transgenic SOD1WT and nontransgenic mice. The membranes were also probed for the ER resident protein calnexin as internal standard and β-actin. (B) Immunoblot of cerebellar microsomal fractions from mice of the different genotypes. (C) Quantification of microsomal SOD1 content over the course of disease relative to calnexin by optical density analysis (n = three to six per group; one-way ANOVA; ∗, P < 0.01; ∗∗, _P_ < 0.05). (_D_) Comparison of microsomal SOD1 content in spinal cord and cerebellum. In nontransgenic and transgenic SOD1WT mice, there is no difference in the SOD1:calnexin ratios between spinal and cerebellar microsomal fractions (_P_ > 0.05). In transgenic SOD1G93A mice, the microsomal SOD1:calnexin ratios are higher in the spinal cord than in the cerebellum (n = three to five per group, two-way ANOVA, Student–Newman–Keuls test; ∗, P < 0.001). SOD1:calnexin ratios in microsomal fractions from transgenic SOD1WT and SOD1G93A mice are higher than in nontransgenic mice in both areas (n = three to five per group; two-way ANOVA; Student—Newman–Keuls method; ∗∗, P < 0.05). (E) SOD1 is protected from surface biotinylation under conditions of intact microsomal membranes. Luminal protein BiP is used as positive control. Lane 1, total input; lane 2, eluate from intact membranes. Little SOD1 and no BiP are biotinylated in the absence of detergent. Lane 3, flow-through from intact membrane. Abundant SOD1 and BiP are found among the nonbiotinylated proteins. Lane 4, eluate from solubilized membranes. Both SOD1 and BiP are now biotinylated. (F) SOD1 is protected from proteinase K proteolysis in intact microsomal membranes and digested after addition of detergent. The mSOD1 is resistant to proteolysis in any condition (lower band).
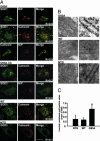
Fig. 3.
Microscopical demonstration of ER location of SOD1 in spinal motor neurons. (A) Immunoflourescence analysis of SOD1 colocalization with ER markers calnexin and BiP in spinal cord and cerebellar sections. (Scale bar, 20 μm.) (B) Luminal ER labeling of SOD1 in immunoelectron microscopy. (Scale bar, 50 nm.) (C) Quantification of gold particles inside the ER lumen and in the ER periphery. Values represent means ± SEM (n = 23–30 ER profiles per group). ∗, P < 0.05; one-way ANOVA, Student–Newman–Keuls method.
Fig. 4.
High molecular weight aggregates of mutant SOD1 in spinal microsomal fractions. (A) Age-dependent increase of high molecular weight SOD1 species in SOD1G93A spinal microsomal fractions in overexposed immunoblot of spinal microsomal fractions of SOD1G93A mice. Asterisks indicate high molecular weight species. (B) Quantification of high molecular weight species over the course of the disease relative to the ER resident protein calnexin by optical density analysis. Values represent means ± SEM (n = three per group). ∗, P < 0.05; one-way ANOVA, Student–Newman–Keuls method.
Fig. 5.
Mutant SOD1, but not hSOD1WT or mSOD1, interacts with BiP in the microsomal fraction in vivo. (A) Immunoprecipitation with an anti-BiP antibody followed by immunoblot using anti-BiP and anti-SOD1 antibodies. (B) Immunoprecipitation with an anti-BiP antibody using spinal microsomal fractions of SOD1G93A mice at different disease stages and nontransgenic mice. Input represents 10% of protein from spinal microsomal fraction. (C) Immunoprecipitation with an anti-BiP antibody followed by immunoblot using anti-BiP, -SOD1, and -ATF6 antibodies. (D) High molecular weight (HMW) SOD1 species in microsomal fractions interact with BiP. (E) Immunoprecipitated with/without anti-BiP antibody followed by a size-exclusion filter assay.
Similar articles
- The endoplasmic reticulum-Golgi pathway is a target for translocation and aggregation of mutant superoxide dismutase linked to ALS.
Urushitani M, Ezzi SA, Matsuo A, Tooyama I, Julien JP. Urushitani M, et al. FASEB J. 2008 Jul;22(7):2476-87. doi: 10.1096/fj.07-092783. Epub 2008 Mar 12. FASEB J. 2008. PMID: 18337461 - Mutant SOD1 linked to familial amyotrophic lateral sclerosis, but not wild-type SOD1, induces ER stress in COS7 cells and transgenic mice.
Tobisawa S, Hozumi Y, Arawaka S, Koyama S, Wada M, Nagai M, Aoki M, Itoyama Y, Goto K, Kato T. Tobisawa S, et al. Biochem Biophys Res Commun. 2003 Apr 4;303(2):496-503. doi: 10.1016/s0006-291x(03)00353-x. Biochem Biophys Res Commun. 2003. PMID: 12659845 - Superoxide dismutase 1 mutants related to amyotrophic lateral sclerosis induce endoplasmic stress in neuro2a cells.
Oh YK, Shin KS, Yuan J, Kang SJ. Oh YK, et al. J Neurochem. 2008 Feb;104(4):993-1005. doi: 10.1111/j.1471-4159.2007.05053.x. J Neurochem. 2008. PMID: 18233996 - Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation.
Shibata N. Shibata N. Neuropathology. 2001 Mar;21(1):82-92. doi: 10.1046/j.1440-1789.2001.00361.x. Neuropathology. 2001. PMID: 11304046 Review. - Stress signaling from the endoplasmic reticulum: A central player in the pathogenesis of amyotrophic lateral sclerosis.
Walker AK, Atkin JD. Walker AK, et al. IUBMB Life. 2011 Sep;63(9):754-63. doi: 10.1002/iub.520. Epub 2011 Aug 10. IUBMB Life. 2011. PMID: 21834058 Review.
Cited by
- Rodent models of TDP-43: recent advances.
Tsao W, Jeong YH, Lin S, Ling J, Price DL, Chiang PM, Wong PC. Tsao W, et al. Brain Res. 2012 Jun 26;1462:26-39. doi: 10.1016/j.brainres.2012.04.031. Epub 2012 May 1. Brain Res. 2012. PMID: 22608070 Free PMC article. Review. - Extracellular wildtype and mutant SOD1 induces ER-Golgi pathology characteristic of amyotrophic lateral sclerosis in neuronal cells.
Sundaramoorthy V, Walker AK, Yerbury J, Soo KY, Farg MA, Hoang V, Zeineddine R, Spencer D, Atkin JD. Sundaramoorthy V, et al. Cell Mol Life Sci. 2013 Nov;70(21):4181-95. doi: 10.1007/s00018-013-1385-2. Epub 2013 Jun 14. Cell Mol Life Sci. 2013. PMID: 23765103 Free PMC article. - Activation of HIPK2 Promotes ER Stress-Mediated Neurodegeneration in Amyotrophic Lateral Sclerosis.
Lee S, Shang Y, Redmond SA, Urisman A, Tang AA, Li KH, Burlingame AL, Pak RA, Jovičić A, Gitler AD, Wang J, Gray NS, Seeley WW, Siddique T, Bigio EH, Lee VM, Trojanowski JQ, Chan JR, Huang EJ. Lee S, et al. Neuron. 2016 Jul 6;91(1):41-55. doi: 10.1016/j.neuron.2016.05.021. Epub 2016 Jun 16. Neuron. 2016. PMID: 27321923 Free PMC article. - The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice.
Wu DC, Ré DB, Nagai M, Ischiropoulos H, Przedborski S. Wu DC, et al. Proc Natl Acad Sci U S A. 2006 Aug 8;103(32):12132-7. doi: 10.1073/pnas.0603670103. Epub 2006 Jul 28. Proc Natl Acad Sci U S A. 2006. PMID: 16877542 Free PMC article. - The unfolded protein response in familial amyotrophic lateral sclerosis.
Wang L, Popko B, Roos RP. Wang L, et al. Hum Mol Genet. 2011 Mar 1;20(5):1008-15. doi: 10.1093/hmg/ddq546. Epub 2010 Dec 15. Hum Mol Genet. 2011. PMID: 21159797 Free PMC article.
References
- Julien J. P. Cell. 2001;104:581–591. - PubMed
- Bruijn L. I., Houseweart M. K., Kato S., Anderson K. L., Anderson S. D., Ohama E., Reaume A. G., Scott R. W., Cleveland D. W. Science. 1998;281:1851–1854. - PubMed
- Bruijn L. I., Becher M. W., Lee M. K., Anderson K. L., Jenkins N. A., Copeland N. G., Sisodia S., Rothstein J. D., Borchelt D. R., Price D. L., et al. Neuron. 1997;18:327–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS38586/NS/NINDS NIH HHS/United States
- R01 AG021617/AG/NIA NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- P01 NS11766-27/NS/NINDS NIH HHS/United States
- P50 NS38370/NS/NINDS NIH HHS/United States
- R01 AG21617-01/AG/NIA NIH HHS/United States
- R21 ES013177/ES/NIEHS NIH HHS/United States
- P01 NS011766/NS/NINDS NIH HHS/United States
- R01 NS042269/NS/NINDS NIH HHS/United States
- R01 NS038586/NS/NINDS NIH HHS/United States
- NS42269/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous