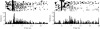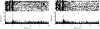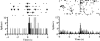Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex - PubMed (original) (raw)
Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex
George V Rebec et al. J Exp Anal Behav. 2005 Nov.
Abstract
The return to drug seeking, even after prolonged periods of abstinence, is a defining feature of cocaine addiction. The neural circuitry underlying relapse has been identified in neuropharmacological studies of experimental animals, typically rats, and supported in brain imaging studies of human addicts. Although the nucleus accumbens (NAcc), which has long been implicated in goal-directed behavior, plays a critical role in this circuit, the prefrontal cortex (PFC) appears to process the events that directly trigger relapse: exposure to acute stress, cues previously associated with the drug, and the drug itself. In this paper, we review animal models of relapse and what they have revealed about the mechanisms underlying the involvement of the NAcc and PFC in cocaine-seeking behavior. We also present electrophysiological data from PFC illustrating how the hedonic, motor, motivational, and reinforcing effects of cocaine can be analyzed at the neuronal level. Our preliminary findings suggest a role for PFC in processing information related to cocaine seeking but not the hedonic effects of the drug. Further use of this recording technology can help dissect the functions of PFC and other components of the neural circuitry underlying relapse.
Figures
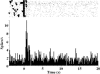
Figure 1. Cue-related activation of neuronal activity in PFC (Cg1–PLC) of one rat during cocaine self-administration.
The upper part of the figure is a raster display in which each dot represents a spike from the recorded neuron and each row represents an individual trial. Black triangles indicate lever presses; lever presses that occur at Time 0 trigger cue presentation and activation of the pump for cocaine infusion. The PETH in the lower part of the figure represents the average firing rate around the time of cue onset. The Y axis depicts firing rate (spikes /s); bin size is 100 ms. Note the increase in firing rate that begins within 500 ms of cue onset and that persists for ∼700 ms.
Figure 2. PFC (Cg1–PLC) activity during a cocaine self-administration session in which the cue is omitted on a random 50% of trials.
Data are presented as in Figure 1. Left: Trials with cue. Note that as in Figure 1, the transient activation of neuronal activity was within 500 ms after cue onset. Right: Trials without cue. Note the complete absence of neuronal activation during the same period after cue onset. Interestingly, a late period of activation emerged on trials without cue at the time of houselight onset (4 s after cue onset).
Figure 3. PFC (Cg1–PLC) activity during a cocaine self-administration session in which the cocaine infusion is omitted on a random 50% of trials.
Data are presented as in Figure 1. Left: Trials with cocaine infusion (infusion pump on from 0–4 s). Note the brief activation of unit activity almost immediately after cue onset. Right: Trials without cocaine infusion. Note the similarity of the left and right PETHs throughout the 20-s period after cue onset.
Figure 4. PFC (Cg1–PLC) activity during conditioned (left) and cue-primed (right) reinstatement.
Data are presented as in Figure 1, but bin size is 50 ms. In both graphs, transient, cue-related activation is evident within 500 ms after cue onset. Over the course of both reinstatement sessions, lever pressing (black triangles) exceeded that during extinction by 4 times (left; a total of 36 lever presses; 13 are shown) and 12 times (right; a total of 20 lever presses; one is shown). During conditioned reinstatement, cue presentation follows an FR-5 schedule.
Similar articles
- Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior.
McFarland K, Davidge SB, Lapish CC, Kalivas PW. McFarland K, et al. J Neurosci. 2004 Feb 18;24(7):1551-60. doi: 10.1523/JNEUROSCI.4177-03.2004. J Neurosci. 2004. PMID: 14973230 Free PMC article. - A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats.
Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW Jr, Miller SW, McGinty JF. Berglind WJ, et al. Eur J Neurosci. 2007 Aug;26(3):757-66. doi: 10.1111/j.1460-9568.2007.05692.x. Epub 2007 Jul 25. Eur J Neurosci. 2007. PMID: 17651427 - Increased accumbens Cdk5 expression in rats after short-access to self-administered cocaine, but not after long-access sessions.
Seiwell AP, Reveron ME, Duvauchelle CL. Seiwell AP, et al. Neurosci Lett. 2007 Apr 24;417(1):100-5. doi: 10.1016/j.neulet.2007.02.043. Epub 2007 Feb 20. Neurosci Lett. 2007. PMID: 17339080 Free PMC article. - Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors.
Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Weiss F, et al. Ann N Y Acad Sci. 2001 Jun;937:1-26. doi: 10.1111/j.1749-6632.2001.tb03556.x. Ann N Y Acad Sci. 2001. PMID: 11458532 Review. - Relapse to drug-seeking: neural and molecular mechanisms.
Self DW, Nestler EJ. Self DW, et al. Drug Alcohol Depend. 1998 Jun-Jul;51(1-2):49-60. doi: 10.1016/s0376-8716(98)00065-9. Drug Alcohol Depend. 1998. PMID: 9716929 Review.
Cited by
- The encoding of cocaine vs. natural rewards in the striatum of nonhuman primates: categories with different activations.
Opris I, Hampson RE, Deadwyler SA. Opris I, et al. Neuroscience. 2009 Sep 29;163(1):40-54. doi: 10.1016/j.neuroscience.2009.06.002. Epub 2009 Jun 6. Neuroscience. 2009. PMID: 19501630 Free PMC article. - Expanding the Scope: Beyond the Familiar and Beyond the Page.
Hantula DA. Hantula DA. Behav Anal. 2016 Oct 7;39(2):189-196. doi: 10.1007/s40614-016-0078-1. eCollection 2016 Oct. Behav Anal. 2016. PMID: 31976940 Free PMC article. No abstract available. - Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex.
Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, Szumlinski KK. Shin CB, et al. Neuropharmacology. 2016 Mar;102:103-10. doi: 10.1016/j.neuropharm.2015.10.038. Epub 2015 Oct 30. Neuropharmacology. 2016. PMID: 26522436 Free PMC article. - Activators of G-protein signaling 3: a drug addiction molecular gateway.
Bowers MS. Bowers MS. Behav Pharmacol. 2010 Sep;21(5-6):500-13. doi: 10.1097/FBP.0b013e32833dcfa5. Behav Pharmacol. 2010. PMID: 20700046 Free PMC article. Review. - The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration.
LaLumiere RT, Niehoff KE, Kalivas PW. LaLumiere RT, et al. Learn Mem. 2010 Mar 23;17(4):168-75. doi: 10.1101/lm.1576810. Print 2010 Apr. Learn Mem. 2010. PMID: 20332188 Free PMC article.
References
- Anderson S.M, Bari A.A, Pierce R.C. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. - PubMed
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Experimental Brain Research. 1991;85:491–500. - PubMed
- Baker D.A, McFarland K, Lake R.W, Shen H, Tang X.C, Toda S, Kalivas P.W. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature Neuroscience. 2003;6:743–749. - PubMed
- Bergman J, Katz J.L. Behavioral pharmacology of cocaine and the determinants of abuse liability. In: Higgins S.T, Katz J.L, editors. Cocaine abuse: Behavior, pharmacology, and clinical applications. San Diego: Academic Press; 1998. pp. 51–79.
- Bowers M.S, Lake R.W, McFarland K, Peterson Y.K, Lanier S.M, Lapish C.C, Kalivas P.W. AGS3: A G-Protein regulator of addiction-associated behaviors. Annals of the New York Academy of Science. 2003;1003:356–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous