Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients - PubMed (original) (raw)
Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients
H E Innes et al. Br J Cancer. 2006.
Abstract
The anterior gradient protein-2 (AGR2) is inducible by oestrogen and itself can induce metastasis in a rat model for breast cancer. Here, a rabbit antibody to recombinant human AGR2 was used to assess its prognostic significance in a retrospective cohort of 351 breast cancer patients treated by adjuvant hormonal therapy. The antibody stains 66% of breast carcinomas to varying degrees. The percentage of positive carcinoma cells in tumours directly correlates with the level of AGR2 mRNA (Spearman's rank correlation, P = 0.0007) and protein (linear regression analysis r2 = 0.95, P = 0.0002). There is a significant association of staining of carcinomas for AGR2 with oestrogen receptor alpha (ERalpha) staining and with low histological grade (both Fisher's Exact test P<0.0001). In the ERalpha-positive cases, but not the ERalpha-negative cases, when subdivided into the separate staining classes for AGR2, there is a significantly progressive decrease in patient survival with increased staining (log rank test, P = 0.006). The significant association of staining for AGR2 with patient death over a 10-year period (log rank test P = 0.007, hazard ratio = 3) only becomes significant at 6 years of follow-up. This may be due to the cessation of adjuvant hormonal therapy at an earlier time, resulting in adverse re-expression of the metastasis-inducing protein AGR2.
Figures
Figure 1
Immunocytochemical staining for AGR2. Antiserum to AGR2 was incubated with histological sections of specimens from (A) normal breast or from (B–D) primary tumours of different breast carcinomas showing: (B) unstained (−); (C) borderline (±); (D) strong immunocytochemical staining of the carcinoma cells (+++). Arrows point to the occasional stained cell in C; n is unstained normal breast tissue in D. (E) is a higher magnification of D showing strong immunocytochemical staining of the cytoplasm (arrowheads) and membranous region (arrows) of the carcinoma cells. (F) Antiserum to AGR2 preincubated with pure human rAGR2 applied to an adjacent serial section to that in E showing no immunocytochemical staining. (G) Antiserum to AGR2 preincubated with pure human rAGR2 showing no immunocytochemical staining over a larger field at lower magnification to that in F. (H) Antiserum to AGR2 preincubated with pure human rAGR3 showing strong immunocytochemical staining of the carcinoma cells in a section adjacent to that in G, the normal breast tissue n is unstained. Magnification A–D, G, H × 230; E, F × 580. Bars A–D, G, H 50 _μ_m; E, F 20 _μ_m.
Figure 2
Detection of AGR2 by Western blotting and RT–PCR. (A) Protein samples (20 _μ_g) from invasive carcinomas of the following classes of immunocytochemical staining for AGR2: unstained (−; lane 1), borderline (±; lanes 2 and 3), moderate (++; lane 4), strong (+++; lane 5), very strong (++++; lanes 6 and 7), and 10 _μ_g of protein from human breast cancer cell line, MCF-7 (lane 8) or 0.5 _μ_g of purified His-tagged rAGR2 (lane 9) were subjected to polyacrylamide gel electrophoresis and blotted onto Immobilon PVDF membranes as described in Materials and Methods. The membranes were incubated overnight at 4°C with one of the following: a 1 : 400 dilution of rabbit polyclonal anti-human AGR2 (A, upper panel), the same amount of rabbit polyclonal anti-human AGR2 preincubated overnight at 4°C with 1 mg ml−1 human rAGR2 (A, middle panel) and 1 : 1000 dilution of rabbit anti-_β_-actin antibodies (A, lower panel). The positions of human natural AGR2 (nAGR2), recombinant His-tagged AGR2 (rAGR2) and actin are shown on the right-hand side of the panels and those of molecular weight markers are shown on the left-hand side of the panels. (B) 5 _μ_g (B, SDS-PAGE panel) or 0.5 _μ_g (B, Western blot panels) of purified His-tagged rAGR2 and His-tagged rAGR3 were subjected to 12.5% (w v−1) polyacrylamide SDS gel electrophoresis and stained with Coomassie blue G250 (B, SDS-PAGE panel) or blotted onto an Immobilon PVDF membrane (B, Western blot panels) as described in Materials and Methods. The membrane was incubated with one of the following: a 1 : 500 dilution of rabbit polyclonal anti-human AGR2 (B, Western blot 1 (AGR2 Ab) panel), the same amount of rabbit polyclonal anti-human AGR2 preincubated overnight at 4°C with 1 mg ml−1 human rAGR3 protein (B, Western blot 2 (blocked with rAGR3) panel), and the bound antibodies visualized as above. The positions of rAGR2 and rAGR3 are shown on the right-hand side of the panel and those of molecular weight markers are shown on the left-hand side. (C) Relative expression of AGR2 mRNA is shown in four categories of breast tumours: a, patients with carcinomas classified as unstained (−); b, borderline (±); c, intermediate (+) or d, moderate to very strong staining for AGR2 (++, +++ or ++++). AGR2 mRNA was determined by qRT–PCR and is shown relative to that in MCF-7 cells (_δδ_Ct relative to MCF-7) as described in Materials and Methods. The box represents the interquartile range, the line across the box indicates the median and the whiskers extend from the box to the highest and lowest values (excluding outliers and extreme points).
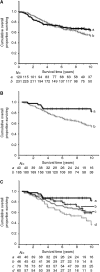
Figure 3
Association of immunocytochemical staining for AGR2 with overall survival of patients using a 1% cutoff between the two staining classes for (A) all cases, (B) ER_α_-positive cases or (C) by degree of immunostaining for AGR2 in ER_α_-positive cases. In (A and B), the cumulative proportion of surviving patients as a fraction of the total for each year after presentation for either a patients with carcinomas classified as negatively staining (black, unbroken line) or b positively staining (grey broken line) for AGR2 is shown. In (A), there were 81 censored observations in a and 139 in b. The cumulative proportions surviving were a, 0.71 at 5 years (standard error (s.e.)=0.04) and 0.64 at 10 years (s.e.=0.05) and b, 0.69 at 5 years (s.e.=0.06) and 0.54 at 10 years (s.e.=0.04). In (B), there were 34 censored observation in a and 114 in b. The cumulative proportions surviving were a 0.87 at 5 years (s.e.=0.05) and 0.82 at 10 years (s.e.=0.07) and b 0.81 at 5 years (s.e.=0.06) and 0.60 at 10 years (s.e.=0.09). In (C), the cumulative proportion of surviving patients with ER_α_ positive primary tumours for a patients with carcinoma cells classified as unstained (black, unbroken line), b borderline (±, black broken line), c intermediate (+, grey unbroken line) or d moderate to very strong staining for AGR2 (++, +++ or ++++, grey broken line) is shown. There were 34 censored observations in a; 32 in b; 50 in c and 32 in d. The cumulative proportions surviving were a 0.87 (s.e.=0.05); b 0.81 (s.e.=0.06), c 0.73 (s.e.=0.05) and d 0.59 (s.e.=0.07) at 5 years; and a 0.82 (s.e.=0.07); b 0.60 (s.e.=0.09); c 0.59 (s.e.=0.06) and d 0.39 (s.e.=0.09) at 10 years. In all three panels, censored observations are denoted by vertical lines and the number of surviving patients in each subgroup (N) at 12 monthly intervals is shown below each panel.
Similar articles
- [Expression of a novel metastasis-inducing protein human anterior gradient-2 (AGR2) in breast cancer and its clinical and prognostic significance].
Wu ZS, Wu Q, Ding XD, Wang HQ, Shen YX, Fang SY. Wu ZS, et al. Zhonghua Bing Li Xue Za Zhi. 2008 Feb;37(2):109-13. Zhonghua Bing Li Xue Za Zhi. 2008. PMID: 18681322 Chinese. - Association of oestrogen receptor beta 2 (ER beta 2/ER beta cx) with outcome of adjuvant endocrine treatment for primary breast cancer--a retrospective study.
Vinayagam R, Sibson DR, Holcombe C, Aachi V, Davies MP. Vinayagam R, et al. BMC Cancer. 2007 Jul 18;7:131. doi: 10.1186/1471-2407-7-131. BMC Cancer. 2007. PMID: 17640362 Free PMC article. - Anterior Gradient 2 is a Poor Outcome Indicator in Luminal Breast Cancer.
Lacambra MD, Tsang JY, Ni YB, Chan SK, Tan PH, Tse GM. Lacambra MD, et al. Ann Surg Oncol. 2015 Oct;22(11):3489-96. doi: 10.1245/s10434-015-4420-8. Epub 2015 Feb 7. Ann Surg Oncol. 2015. PMID: 25663596 - The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: a potential drug target and biomarker.
Salmans ML, Zhao F, Andersen B. Salmans ML, et al. Breast Cancer Res. 2013 Apr 24;15(2):204. doi: 10.1186/bcr3408. Breast Cancer Res. 2013. PMID: 23635006 Free PMC article. Review. - Anterior gradient 2: a novel player in tumor cell biology.
Brychtova V, Vojtesek B, Hrstka R. Brychtova V, et al. Cancer Lett. 2011 May 1;304(1):1-7. doi: 10.1016/j.canlet.2010.12.023. Epub 2011 Mar 2. Cancer Lett. 2011. PMID: 21371820 Review.
Cited by
- Induction of anterior gradient 2 (AGR2) plays a key role in insulin-like growth factor-1 (IGF-1)-induced breast cancer cell proliferation and migration.
Li Z, Wu Z, Chen H, Zhu Q, Gao G, Hu L, Negi H, Kamle S, Li D. Li Z, et al. Med Oncol. 2015 Jun;32(6):577. doi: 10.1007/s12032-015-0577-z. Epub 2015 May 9. Med Oncol. 2015. PMID: 25956506 Free PMC article. - The Utility of SOX2 and AGR2 Biomarkers as Early Predictors of Tamoxifen Resistance in ER-Positive Breast Cancer Patients.
Zamzam Y, Abdelmonem Zamzam Y, Aboalsoud M, Harras H. Zamzam Y, et al. Int J Surg Oncol. 2021 Sep 15;2021:9947540. doi: 10.1155/2021/9947540. eCollection 2021. Int J Surg Oncol. 2021. PMID: 34567804 Free PMC article. - Anterior gradient protein 3 is associated with less aggressive tumors and better outcome of breast cancer patients.
Obacz J, Brychtova V, Podhorec J, Fabian P, Dobes P, Vojtesek B, Hrstka R. Obacz J, et al. Onco Targets Ther. 2015 Jun 24;8:1523-32. doi: 10.2147/OTT.S82235. eCollection 2015. Onco Targets Ther. 2015. PMID: 26170690 Free PMC article. - ErbB3 binding protein 1 represses metastasis-promoting gene anterior gradient protein 2 in prostate cancer.
Zhang Y, Ali TZ, Zhou H, D'Souza DR, Lu Y, Jaffe J, Liu Z, Passaniti A, Hamburger AW. Zhang Y, et al. Cancer Res. 2010 Jan 1;70(1):240-8. doi: 10.1158/0008-5472.CAN-09-2904. Cancer Res. 2010. PMID: 20048076 Free PMC article. - Leveraging the Role of the Metastatic Associated Protein Anterior Gradient Homologue 2 in Unfolded Protein Degradation: A Novel Therapeutic Biomarker for Cancer.
Alsereihi R, Schulten HJ, Bakhashab S, Saini K, Al-Hejin AM, Hussein D. Alsereihi R, et al. Cancers (Basel). 2019 Jun 26;11(7):890. doi: 10.3390/cancers11070890. Cancers (Basel). 2019. PMID: 31247903 Free PMC article. Review.
References
- Altman DG (1991) Practical Statistics for Medical Research. London: Chapman and Hall
- Clarke R, Dickson RB, Brümer N (1990) The process of malignant progression in human breast cancer. Ann Oncol 1: 401–407 - PubMed
- Clarke R, Thompson EW, Leonessa F, Lippman J, McGarvey M, Frandsen TL, Brünner N (1994) Hormone resistance, invasiveness and metastatic potential in breast cancer. Br Cancer Res Treat 24: 227–239 - PubMed
- Davies BR, Davies MP, Gibbs FE, Barraclough R, Rudland PS (1993) Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for p9Ka, a rat calcium-binding protein, but not with the oncogene EJ-ras-1. Oncogene 8: 999–1008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous