What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies - PubMed (original) (raw)
What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies
Alison M McDonald et al. Trials. 2006.
Abstract
Background: A commonly reported problem with the conduct of multicentre randomised controlled trials (RCTs) is that recruitment is often slower or more difficult than expected, with many trials failing to reach their planned sample size within the timescale and funding originally envisaged. The aim of this study was to explore factors that may have been associated with good and poor recruitment in a cohort of multicentre trials funded by two public bodies: the UK Medical Research Council (MRC) and the Health Technology Assessment (HTA) Programme.
Methods: The cohort of trials was identified from the administrative databases held by the two funding bodies. 114 trials that recruited participants between 1994 and 2002 met the inclusion criteria. The full scientific applications and subsequent trial reports submitted by the trial teams to the funders provided the principal data sources. Associations between trial characteristics and recruitment success were tested using the Chi-squared test, or Fisher's exact test where appropriate.
Results: Less than a third (31%) of the trials achieved their original recruitment target and half (53%) were awarded an extension. The proportion achieving targets did not appear to improve over time. The overall start to recruitment was delayed in 47 (41%) trials and early recruitment problems were identified in 77 (63%) trials. The inter-relationship between trial features and recruitment success was complex. A variety of strategies were employed to try to increase recruitment, but their success could not be assessed.
Conclusion: Recruitment problems are complex and challenging. Many of the trials in the cohort experienced recruitment difficulties. Trials often required extended recruitment periods (sometimes supported by additional funds). While this is of continuing concern, success in addressing the trial question may be more important than recruitment alone.
Figures
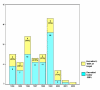
Figure 1
Recruitment success related to year trial commenced.
Similar articles
- Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme.
Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, Knox C, Nadin B, Rothwell J, Surtees M, Julious SA. Walters SJ, et al. BMJ Open. 2017 Mar 20;7(3):e015276. doi: 10.1136/bmjopen-2016-015276. BMJ Open. 2017. PMID: 28320800 Free PMC article. Review. - A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies.
Sully BG, Julious SA, Nicholl J. Sully BG, et al. Trials. 2013 Jun 9;14:166. doi: 10.1186/1745-6215-14-166. Trials. 2013. PMID: 23758961 Free PMC article. Review. - Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study.
Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, Entwistle V, Garcia J, Roberts I, Grant A, Grant A; STEPS group. Campbell MK, et al. Health Technol Assess. 2007 Nov;11(48):iii, ix-105. doi: 10.3310/hta11480. Health Technol Assess. 2007. PMID: 17999843 Review. - Carer administration of as-needed subcutaneous medication for breakthrough symptoms in people dying at home: the CARiAD feasibility RCT.
Poolman M, Roberts J, Wright S, Hendry A, Goulden N, Holmes EA, Byrne A, Perkins P, Hoare Z, Nelson A, Hiscock J, Hughes D, O'Connor J, Foster B, Reymond L, Healy S, Lewis P, Wee B, Johnstone R, Roberts R, Parkinson A, Roberts S, Wilkinson C. Poolman M, et al. Health Technol Assess. 2020 May;24(25):1-150. doi: 10.3310/hta24250. Health Technol Assess. 2020. PMID: 32484432 Free PMC article. Clinical Trial. - The future of Cochrane Neonatal.
Soll RF, Ovelman C, McGuire W. Soll RF, et al. Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
Cited by
- Recruitment patterns in a large international randomized controlled trial of perioperative care in cancer patients.
Gazendam A, Bozzo A, Schneider P, Giglio V, Wilson D, Ghert M. Gazendam A, et al. Trials. 2021 Mar 20;22(1):219. doi: 10.1186/s13063-021-05149-0. Trials. 2021. PMID: 33743753 Free PMC article. Clinical Trial. - Recruiting and retaining postpartum women from areas of social disadvantage in a weight-loss trial--an assessment of strategies employed in the WeighWell feasibility study.
Macleod M, Craigie AM, Barton KL, Treweek S, Anderson AS; WeighWell team. Macleod M, et al. Matern Child Nutr. 2013 Jul;9(3):322-31. doi: 10.1111/j.1740-8709.2011.00393.x. Epub 2012 Jan 29. Matern Child Nutr. 2013. PMID: 22284216 Free PMC article. Clinical Trial. - Development of the ehive Digital Health App: Protocol for a Centralized Research Platform.
Hirten RP, Danieletto M, Landell K, Zweig M, Golden E, Orlov G, Rodrigues J, Alleva E, Ensari I, Bottinger E, Nadkarni GN, Fuchs TJ, Fayad ZA. Hirten RP, et al. JMIR Res Protoc. 2023 Nov 16;12:e49204. doi: 10.2196/49204. JMIR Res Protoc. 2023. PMID: 37971801 Free PMC article. - Deep Learning Approach to Parse Eligibility Criteria in Dietary Supplements Clinical Trials Following OMOP Common Data Model.
Bompelli A, Li J, Xu Y, Wang N, Wang Y, Adam T, He Z, Zhang R. Bompelli A, et al. AMIA Annu Symp Proc. 2021 Jan 25;2020:243-252. eCollection 2020. AMIA Annu Symp Proc. 2021. PMID: 33936396 Free PMC article. - How informed is declared altruism in clinical trials? A qualitative interview study of patient decision-making about the QUEST trials (Quality of Life after Mastectomy and Breast Reconstruction).
Bidad N, MacDonald L, Winters ZE, Edwards SJ, Emson M, Griffin CL, Bliss J, Horne R. Bidad N, et al. Trials. 2016 Sep 2;17(1):431. doi: 10.1186/s13063-016-1550-7. Trials. 2016. PMID: 27590594 Free PMC article.
References
- Pocock S. Clinical trials: a practical approach. Chichester: Wiley; 1983.
- Bowling A. Research methods in health: investigating health and health services. 2. Buckingham, Philadelphia: Open University Press; 2002.
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the number, quality and progress of randomised controlled trials. Health Technol Assess. 1999;3 - PubMed
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald A, Knight R, Entwistle V, Garcia J, Roberts I, Grant A. Recruitment to randomised trials: Strategies for Trial Enrolment and Participation Study (STEPS) Methodology Series. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous