B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma - PubMed (original) (raw)
. 2006 Apr 17;203(4):871-81.
doi: 10.1084/jem.20050930. Epub 2006 Apr 10.
Linhua Zou, Paulo Rodriguez, Gefeng Zhu, Shuang Wei, Peter Mottram, Michael Brumlik, Pui Cheng, Tyler Curiel, Leann Myers, Andrew Lackner, Xavier Alvarez, Augusto Ochoa, Lieping Chen, Weiping Zou
Affiliations
- PMID: 16606666
- PMCID: PMC2118300
- DOI: 10.1084/jem.20050930
B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma
Ilona Kryczek et al. J Exp Med. 2006.
Abstract
Tumor-associated macrophages are a prominent component of ovarian cancer stroma and contribute to tumor progression. B7-H4 is a recently identified B7 family molecule. We show that primary ovarian tumor cells express intracellular B7-H4, whereas a fraction of tumor macrophages expresses surface B7-H4. B7-H4+ tumor macrophages, but not primary ovarian tumor cells, suppress tumor-associated antigen-specific T cell immunity. Blocking B7-H4-, but not arginase-, inducible nitric oxide synthase or B7-H1 restored the T cell stimulating capacity of the macrophages and contributes to tumor regression in vivo. Interleukin (IL)-6 and IL-10 are found in high concentrations in the tumor microenvironment. These cytokines stimulate macrophage B7-H4 expression. In contrast, granulocyte/macrophage colony-stimulating factor and IL-4, which are limited in the tumor microenvironment, inhibit B7-H4 expression. Ectopic expression of B7-H4 makes normal macrophages suppressive. Thus, B7-H4+ tumor macrophages constitute a novel suppressor cell population in ovarian cancer. B7-H4 expression represents a critical checkpoint in determining host responses to dysfunctional cytokines in ovarian cancer. Blocking B7-H4 or depleting B7-H4+ tumor macrophages may represent novel strategies to enhance T cell tumor immunity in cancer.
Figures
Figure 1.
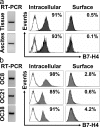
Ovarian tumor cells express intracellular B7-H4. B7-H4 expression was analyzed by RT-PCR and FACS in (a) fresh primary ovarian tumor cells and (b) ovarian tumor cell lines. FACS results expressed the mean percentage of B7-H4–expressing cells. OC8, OC21, and OC38 are three established ovarian tumor cell lines. Fresh primary ovarian tumor cells were isolated and sorted from ascites and tumor tissues with a phenotype of lin−EpCam+CD45−CD14−. Two of six representative patient samples are shown for panel a. Three of eight cell lines are shown for panel b. Filled histogram, B7-H4 expression; open histogram, isotype control.
Figure 2.
Tumor-associated macrophages express B7-H4. (a and b) Tumor ascites macrophages express B7-H4. FACS analysis showed that ascites macrophages, but not control nonmalignant ascites macrophages, or fresh normal blood monocytes expressed B7-H4. Results are expressed as the mean of the percentage of B7-H4+ cells ± SEM in total macrophages. Filled histogram, B7-H4 staining; open histogram, isotype control. (c) High prevalence of B7-H4+ tumor macrophages in tumor ascites. FACS analysis was used to determine the prevalence of B7-H4+ tumor macrophages and CD3+CD4+CD25+ cells in tumor ascites (*, P < 0.001). Results are expressed as the mean of percentage ± SEM in tumor ascites mononuclear cells. (d and e) Tumor tissues were stained with anti–human B7-H4, anti–human CD3, anti–human-Ham56, and control antibody as described in Materials and methods, and analyzed with confocal microscope. (d) Tumor mass macrophages and tumor cells express B7-H4. B7-H4, green; Ham56, red. Tumor macrophages are identified as Ham56+ cells (red). Ham56+B7-H4+ cells are yellowish. Large numbers of tumor macrophages form a barrier surrounding tumor islets. (e) Tumor-infiltrating T cells are B7-H4−. Tumor cells, but not CD3+ T cells (red), expressed B7-H4 (green). 1 of 60 representative patient samples is shown for panels d and e.
Figure 3.
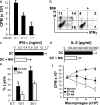
Tumor ascites IL-6 and IL-10 stimulate macrophage B7-H4 expression. (a) Tumor ascites stimulated macrophage B7-H4 expression. Fresh normal blood monocytes were cultured 72 h with ovarian tumor ascites in the presence or absence of anti–human IL-6 or anti–human IL-10 antibodies. B7-H4 expression was analyzed by FACS. Results are expressed as the mean of B7-H4+ cell percentage ± SEM in total macrophages. (b) High concentrations of IL-6 and IL-10 in tumor ascites were detected by ELISA. (c) Recombinant IL-6 or IL-10 stimulated macrophage B7-H4 protein in a dose-dependent manner after a 72 h culture. B7-H4 expression was analyzed by FACS. Results are expressed as the mean of B7-H4+ cell percentage ± SEM in total macrophages. (d and e) Recombinant IL-4 and GM-CSF reduced macrophage B7-H4 mRNA and protein induction. Fresh blood monocytes were cultured with different concentrations of cytokines. (d) B7-H4 mRNA was detected by RT-PCR at 24 h. (e) B7-H4 surface protein was detected by FACS at 72 h. One of seven independent experiments is shown.
Figure 4.
Tumor macrophages suppress TAA-specific T cell immunity in vitro. (a–e) Autologous tumor ascites CD3+CD25− T cells were stimulated with Her-2/neu peptide-loaded MDC (TAA-MDC) as described in Materials and methods. (a) Tumor T cell proliferation was detected by [3H]thymidine incorporation (CPM). Results are expressed as the mean of cpm ± SEM. TAA-specific T cell IFN-γ (b and c) and IL-2 (b and d) production was detected by intracellular staining (FACS) and ELISA. (e) Tumor macrophages inhibited Her-2/neu–specific T cell cytotoxicity. MDC-activated Her-2/neu–specific T cells were effector cells, and Her-2/neu peptide-loaded T2 cells were target cells. Her-2/neu–specific cytotoxicity was determined by FACS (see Materials and methods). Tumor macrophage to TAA-MDC ratio was 1:1 for panels b–e. (f) B7-H4+ tumor macrophages significantly suppress T cell proliferation. B7-H4+ or B7-H4− tumor macrophages were sorted from malignant ascites using a FACSaria. Normal macrophages were from M-CSF–treated normal peripheral blood monocytes. Tumor ascites CD3+ T cells were stimulated with anti-CD3 antibody and blood monocytes for 3 d in the presence of different concentrations of tumor macrophages or normal macrophages. T cell proliferation was detected by [3H]thymidine incorporation. Results are expressed as the mean of cpm ± SEM. TAM, tumor macrophages; macrophage, MΦ.
Figure 5.
Blockade of B7-H4 improved macrophage-mediated T cell activation. (a and b) B7-H4–blocking oligos reduced macrophage B7-H4 expression. Fresh blood monocytes were exposed to B7-H4–blocking oligos and control oligos with medium, tumor ascites, and IL-10. (a) B7-H4 mRNA was detected at 24 h by RT-PCR with serial dilutions of cDNA. (b) B7-H4 protein was detected at 72 h by FACS. One of nine independent experiments is shown. (c and d) Blockade of B7-H4 improved tumor ascites-conditioned macrophage-mediated T cell activation in vitro. Tumor ascites-conditioned macrophages were previously treated with B7-H4–blocking oligos or control oligos and were subsequently subject to activating T cells for 72 h. (c) T cell proliferation was measured by [3H]thymidine incorporation. Results are expressed as the mean of cpm ± SEM. (d) Cell cycle in T cells was analyzed by FACS. Results are expressed as the mean of the percentage of T cells ± SEM in each cycle phase. The ratio between macrophages and T cells was 1:1 for panel d. n = 4–8; *, P < 0.05 compared with control oligos.
Figure 6.
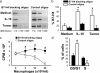
B7-H4+ macrophages mediate an immunosuppression independent of B7-H1, arginase, and iNOS. (a) B7-H1, arginase, and iNOS expression on tumor ascites-associated macrophages. B7-H1 expression was analyzed by FACS. Arginase I and iNOS expression was detected by Western blot. A, B, C, D, and E represent 5 individual patients with ovarian cancer. (b) Tumor ascites-conditioned macrophages mediated T cell suppression through B7-H4. T cells were stimulated with tumor ascites-conditioned macrophages for 72 h in the presence of the indicated conditions. T cell proliferation was measured by [3H]thymidine incorporation in the last 16 h. The results were expressed as the percentage of suppression (mean ± SEM). The ratio between macrophages and T cells was 1:2 for panel d. n = 5; *, P < 0.05.
Figure 7.
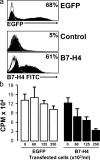
Forced B7-H4 expression confers suppressive capacity of normal macrophages. Fresh blood monocytes were transfected using a plasmid encoding human B7-H4 or EGFP cDNA or control plasmid carried the reversed cDNA sequence of B7-H4. (a) 20 h after transfection, B7-H4 and GFP expression were detected by FACS. One of five experiments is shown. (b) Different concentrations of the transfected cells were added into T cell culture in the presence of anti–human CD3 and normal monocytes. T cell proliferation was detected by [3H]thymidine incorporation on day 3. One of five experiments is shown.
Figure 8.
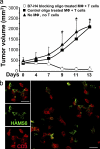
Blockade of B7-H4 improved macrophage-mediated T cell activation in vivo. (a) Blocking tumor ascites-conditioned macrophage B7-H4 results in tumor regression in vivo. Mice were injected with human primary ovarian tumors as described in Materials and methods. Controls received no additional injection (▴, group 1). Treatments were tumor-specific T cells plus B7-H4–blocking oligo-treated macrophages (◯, group 2), and tumor-specific T cells plus control oligo-treated macrophages (▪, group 3). Mean ± SD of tumor volumes is shown (n = 5–7 mice per group). The T cell injection day was counted as day 0. Macrophage, MΦ. (b) Macrophages and T cell were colocalized in tumor tissue. Similar results were observed in group 2 and 3. Macrophages were identified as Ham56+ cells (green) and T cells as CD3+ cells (red). n = 5.
Comment in
- New battlefields for costimulation.
Martin-Orozco N, Dong C. Martin-Orozco N, et al. J Exp Med. 2006 Apr 17;203(4):817-20. doi: 10.1084/jem.20060219. Epub 2006 Apr 10. J Exp Med. 2006. PMID: 16606678 Free PMC article. Review.
Similar articles
- Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma.
Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Kryczek I, et al. Cancer Res. 2007 Sep 15;67(18):8900-5. doi: 10.1158/0008-5472.CAN-07-1866. Cancer Res. 2007. PMID: 17875732 - B7-H4 expression promotes tumorigenesis in ovarian cancer.
Cheng L, Jiang J, Gao R, Wei S, Nan F, Li S, Kong B. Cheng L, et al. Int J Gynecol Cancer. 2009 Dec;19(9):1481-6. doi: 10.1111/IGC.0b013e3181ad0fa2. Int J Gynecol Cancer. 2009. PMID: 19955922 - Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses.
Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, Ra HJ, Danet-Desnoyers G, Powell DJ Jr, Scholler N. Dangaj D, et al. Cancer Res. 2013 Aug 1;73(15):4820-9. doi: 10.1158/0008-5472.CAN-12-3457. Epub 2013 May 30. Cancer Res. 2013. PMID: 23722540 Free PMC article. - Immunopathogenesis of ovarian cancer.
Torres MP, Ponnusamy MP, Lakshmanan I, Batra SK. Torres MP, et al. Minerva Med. 2009 Oct;100(5):385-400. Minerva Med. 2009. PMID: 19910891 Review. - Potential targeting of B7-H4 for the treatment of cancer.
Podojil JR, Miller SD. Podojil JR, et al. Immunol Rev. 2017 Mar;276(1):40-51. doi: 10.1111/imr.12530. Immunol Rev. 2017. PMID: 28258701 Free PMC article. Review.
Cited by
- Immune Tumor Microenvironment in Ovarian Cancer Ascites.
Almeida-Nunes DL, Mendes-Frias A, Silvestre R, Dinis-Oliveira RJ, Ricardo S. Almeida-Nunes DL, et al. Int J Mol Sci. 2022 Sep 14;23(18):10692. doi: 10.3390/ijms231810692. Int J Mol Sci. 2022. PMID: 36142615 Free PMC article. Review. - Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy.
Wang Y, Johnson KCC, Gatti-Mays ME, Li Z. Wang Y, et al. J Hematol Oncol. 2022 Aug 28;15(1):118. doi: 10.1186/s13045-022-01335-y. J Hematol Oncol. 2022. PMID: 36031601 Free PMC article. Review. - Macrophage Biology and Mechanisms of Immune Suppression in Breast Cancer.
Mehta AK, Kadel S, Townsend MG, Oliwa M, Guerriero JL. Mehta AK, et al. Front Immunol. 2021 Apr 23;12:643771. doi: 10.3389/fimmu.2021.643771. eCollection 2021. Front Immunol. 2021. PMID: 33968034 Free PMC article. Review. - Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis.
Tan HY, Wang N, Zhang C, Chan YT, Yuen MF, Feng Y. Tan HY, et al. Hepatology. 2021 Jun;73(6):2326-2341. doi: 10.1002/hep.31600. Epub 2021 May 21. Hepatology. 2021. PMID: 33068461 Free PMC article. - Endogenous interleukin-10 constrains Th17 cells in patients with inflammatory bowel disease.
Wilke CM, Wang L, Wei S, Kryczek I, Huang E, Kao J, Lin Y, Fang J, Zou W. Wilke CM, et al. J Transl Med. 2011 Dec 16;9:217. doi: 10.1186/1479-5876-9-217. J Transl Med. 2011. PMID: 22176654 Free PMC article.
References
- Spiotto, M.T., P. Yu, D.A. Rowley, M.I. Nishimura, S.C. Meredith, T.F. Gajewski, Y.X. Fu, and H. Schreiber. 2002. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 17:737–747. - PubMed
- Yu, P., Y. Lee, W. Liu, R.K. Chin, J. Wang, Y. Wang, A. Schietinger, M. Philip, H. Schreiber, and Y.X. Fu. 2004. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat. Immunol. 5:141–149. - PubMed
- Zou, W. 2005. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 5:263–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA092562/CA/NCI NIH HHS/United States
- CA099985/CA/NCI NIH HHS/United States
- CA97085/CA/NCI NIH HHS/United States
- R21 CA100227/CA/NCI NIH HHS/United States
- R01 CA099985/CA/NCI NIH HHS/United States
- CA100227/CA/NCI NIH HHS/United States
- R01 CA097085/CA/NCI NIH HHS/United States
- CA092562/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials