Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation - PubMed (original) (raw)
Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation
Didrik Paus et al. J Exp Med. 2006.
Abstract
B cells responding to T-dependent antigen either differentiate rapidly into extrafollicular plasma cells or enter germinal centers and undergo somatic hypermutation and affinity maturation. However, the physiological cues that direct B cell differentiation down one pathway versus the other are unknown. Here we show that the strength of the initial interaction between B cell receptor (BCR) and antigen is a primary determinant of this decision. B cells expressing a defined BCR specificity for hen egg lysozyme (HEL) were challenged with sheep red blood cell conjugates of a series of recombinant mutant HEL proteins engineered to bind this BCR over a 10,000-fold affinity range. Decreasing either initial BCR affinity or antigen density was found to selectively remove the extrafollicular plasma cell response but leave the germinal center response intact. Moreover, analysis of competing B cells revealed that high affinity specificities are more prevalent in the extrafollicular plasma cell versus the germinal center B cell response. Thus, the effectiveness of early T-dependent antibody responses is optimized by preferentially steering B cells reactive against either high affinity or abundant epitopes toward extrafollicular plasma cell differentiation. Conversely, responding clones with weaker antigen reactivity are primarily directed to germinal centers where they undergo affinity maturation.
Figures
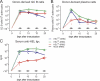
Figure 1.
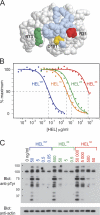
Comparison of the abilities of recombinant HEL proteins to bind to and signal through HyHEL10 in vitro. (A) Rasmol space-filling model of HEL showing the mutated residues (colored) within the HyHEL10-binding footprint (light blue). (B) Competitive ELISA showing relative inhibition of the binding of HELWT-biotin to HyHEL10 by varying concentrations of recombinant HEL proteins. The half-maximal inhibitory concentration for each HEL protein was used to calculate the relative affinities for HyHEL10. (C) Western blot of intracellular tyrosine phosphorylation (pTyr) after 15-min stimulation of SWHEL.rag1 −_/_− B cells with varying concentrations of recombinant HEL proteins. Blue, HELWT; orange, HEL1X (D101HELR); green, HEL2X (R73HELE, D101HELR); red, HEL3X (R21HELQ, R73HELE, and D101HELR).
Figure 2.
SWHEL B cells make in vivo proliferative responses to SRBC conjugates of all three recombinant HEL proteins. SWHEL B cells (CD45.1+) were labeled with CFSE and challenged with various HEL-SRBC conjugates by adoptive transfer into CD45.2+ congenic recipients. (A) Pseudocolor FACS plots show the CFSE profile of donor HEL-binding SWHEL B cells in recipient spleens 64 h after challenge with the various HEL-SRBC conjugates. The data shown were gated from total splenocytes after staining with anti–CD45.1-PE. Numbers indicate the frequency of HEL-binding CFSE+ cells among total splenocytes. Data includes a high frequency of contaminant cells (non-HEL binding and CFSE−) present in this analysis as a result of background staining by the anti–CD45.1-PE antibody. Note the lack of proliferating non-HEL–binding cells, indicating that proliferation is antigen specific and that the HEL-binding stain detects all responding SWHEL donor B cells. (B) CFSE profiles are shown for HEL-binding cells present in the gates shown in A. The frequencies of undivided (CFSEhi) cells are shown. Note the increased frequency of dividing cells and their enhanced rate of proliferation (CFSE dilution) as affinity for the HEL antigen is raised.
Figure 3.
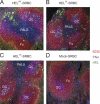
Antigen affinity controls the extrafollicular focus response. SWHEL B cells were adoptively transferred and challenged with either HELWT-SRBC (A), HEL2X-SRBC (B), HEL3X-SRBC (C), or mock-conjugated SRBC (D), and the spleens were harvested at day 5 for immunofluorescence microscopy. B cells in the primary follicle are stained red with B220, GCs stained blue with peanut agglutinin, and HEL-binding Ig stained green with HEL. Anti-HEL plasma cells are evident from their intense cytoplasmic staining with HEL. While HEL-binding B cells formed GCs in response to all three HEL conjugates, no HEL-binding extrafollicular foci of plasma cells were detectable in the spleens of mice challenged with HEL3X-SRBC. EF, extrafollicular focus; GC, germinal center; Fo, follicle; PALS, periarteriolar lymphatic sheath.
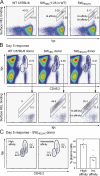
Figure 4.
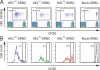
Antigen affinity controls the generation of early plasma cells. Recipient mice were immunized as for Fig. 3 and spleens harvested for FACS analysis on day 5. (A) Surface staining for HEL-binding BCRs and the CD45.2 congenic marker shows two populations of responding donor B cells (CD45.2hi HEL bindinghi vs. CD45.2int HEL bindingint) in mice challenged with HELWT-SRBC and HEL2X-SRBC. Only the CD45.2hi HEL-bindinghi population is observed in mice challenged with HEL3X-SRBC. (B) Cytoplasmic staining for HEL-binding Ig reveals the CD45.2hi populations as low intracellular HEL-binding, syndecan-1− GC B cells, and the CD45.2int population as high intracellular HEL-binding, syndecan-1+ plasma cells (PC). Only rare donor-derived HEL-binding B cells with a plasma cell phenotype were detectable in mice immunized with HEL3X-SRBC. The proportion of spleen cells falling within the different PC and GC gates are indicated.
Figure 5.
Intact GC but impaired extrafollicular focus and antibody responses to low affinity antigen. Recipient mice were immunized as for Fig. 3 and analyzed 5, 10, 15, and 20 d later. (A) Enumeration of donor-derived GC B cells in the spleen by flow cytometry shows similar numbers of GC B cells generated in response to all three HEL conjugates. (B) Enumeration of donor-derived plasma cells shows that the early, transient extrafollicular plasma cell response to HEL3X-SRBC is ∼100-fold lower than those to HELWT-SRBC and HEL2X-SRBC. (C) Serum anti-HELWT Igκ antibody levels were determined by ELISA and also found to be greatly abrogated in mice immunized with HEL3X-SRBC, particularly early in the response (day 5). No anti-HELWT antibody was detectable in control recipients that were immunized with any of the HEL-SRBC conjugates in the absence of cotransferred SWHEL donor cells (not depicted). Each data point represents one mouse. Each data point represents one mouse, whereas bars show the mean of each group. Blue, HELWT-SRBC; green, HEL2X-SRBC; red, HEL3X-SRBC; black, mock-conjugated SRBC.
Figure 6.
Epitope density also controls the extrafollicular plasma cell response and early antibody production. (A) SRBCs were conjugated to HEL2X and HEL3X using 10, 100, or 1,000 μg/ml of recombinant protein to produce low, intermediate, and high epitope density immunogens, respectively. The mean fluorescence intensity (mfi) of HyHEL9 staining is shown in each case. HyHEL9 recognizes both mutant HEL proteins equally well (not depicted). The open curve represents staining of mock-conjugated SRBCs. (B) Spleens from recipient mice were harvested on day 5 and analyzed by flow cytometry for cytoplasmic HEL binding and CD45.2 as for Fig. 4. Gates indicate the plasma cell (CD45.2int, high cytoplasmic HEL binding) and GC (CD45.2hi, low cytoplasmic HEL binding) responding populations, and the proportion of spleen cells in these gates are shown. (C–E) Donor-derived GC, plasma cells, and serum anti-HEL antibody were measured on day 5 of the different responses as for Fig. 5. Points represent data from individual recipients, whereas the bars show the mean of each group. The results show that, at a fixed antigen affinity, early plasma cell and antibody production vary directly according to antigen density, whereas GC responses are relatively unaffected.
Figure 7.
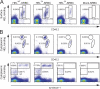
Competing B cells partition into the extrafollicular plasma cell response according to their relative initial antigen affinity. (A) Flow cytometric analysis of lymph node B cells from WT C57BL/6, SWHEL, and SWHEL(H) mice. SWHEL cells were mixed with a 25-fold excess of WT C57BL/6 cells to equalize the frequency of HEL-binding cells between the SWHEL and SWHEL(H) samples. Cells were stained with 75 ng/ml HELWT followed by HyHEL9-AlexaFluor647 and then counterstained for B220 and surface Ig (anti-κ light chain). Data represent B220+ cells. The diagonal gates indicate cells with proportional HELWT binding and surface Ig expression and, therefore, with similar or identical affinity for HELWT. The presence of populations with high and intermediate affinity for HELWT are evident in SWHEL(H) mice. (B) Lymph node cells including 104 HELWT-binding B cells from SWHEL or SWHEL(H) mice were adoptively transferred into CD45.1 congenic recipients and challenged with HELWT-SRBC. Before injection, SWHEL lymph node cells were mixed 1:25 with WT C57BL/6 lymph node cells to ensure the anti-HEL B cells from SWHEL and SWHEL(H) mice comprised similar proportions of the respective donor B cell populations (0.2–0.6%). All recipients including the controls receiving just WT C57BL/6 lymph node cells (left) were therefore injected with ∼2 × 106 non-HELWT–binding B cells each. On day 5, splenocytes from recipient mice were stained with 75 ng/ml HELWT as for A and for CD45.2 and Igκ. Donor B cells were identified with a combination of gates that included CD45.2hi, Igκhi resting plus GC B cells, as well as CD45.2int, Igκint plasma cells. High and intermediate affinity anti-HELWT B cells were identified as for A. (C) Donor B cells with high and intermediate affinity for HELWT from recipients of SWHEL(H) lymph node cells were analyzed separately for the plasma cell (PC) and GC phenotypes. The proportion of high affinity cells exhibiting the CD45.2int Igκint plasma cell phenotype was more than double that evident among the intermediate affinity responders.
Similar articles
- B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells.
Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, Yu D, Fagarasan S, Tarlinton DM, Cunningham AF, Vinuesa CG. Lee SK, et al. J Exp Med. 2011 Jul 4;208(7):1377-88. doi: 10.1084/jem.20102065. Epub 2011 Jun 27. J Exp Med. 2011. PMID: 21708925 Free PMC article. - Expression of the Plasma Cell Transcriptional Regulator Blimp-1 by Dark Zone Germinal Center B Cells During Periods of Proliferation.
Radtke D, Bannard O. Radtke D, et al. Front Immunol. 2019 Jan 9;9:3106. doi: 10.3389/fimmu.2018.03106. eCollection 2018. Front Immunol. 2019. PMID: 30687317 Free PMC article. - Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts.
Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Chan TD, et al. J Immunol. 2009 Sep 1;183(5):3139-49. doi: 10.4049/jimmunol.0901690. Epub 2009 Aug 7. J Immunol. 2009. PMID: 19666691 - Positive Selection in the Light Zone of Germinal Centers.
Nakagawa R, Calado DP. Nakagawa R, et al. Front Immunol. 2021 Mar 31;12:661678. doi: 10.3389/fimmu.2021.661678. eCollection 2021. Front Immunol. 2021. PMID: 33868314 Free PMC article. Review. - BCR Affinity Influences T-B Interactions and B Cell Development in Secondary Lymphoid Organs.
Wishnie AJ, Chwat-Edelstein T, Attaway M, Vuong BQ. Wishnie AJ, et al. Front Immunol. 2021 Jul 26;12:703918. doi: 10.3389/fimmu.2021.703918. eCollection 2021. Front Immunol. 2021. PMID: 34381455 Free PMC article. Review.
Cited by
- Targeting RSV-neutralizing B cell receptors with anti-idiotypic antibodies.
Scharffenberger SC, Wan YH, Homad LJ, Kher G, Haynes AM, Poudel B, Sinha IR, Aldridge N, Pai A, Bibby M, Chhan CB, Davis AR, Moodie Z, Palacio MB, Escolano A, McElrath MJ, Boonyaratanakornkit J, Pancera M, McGuire AT. Scharffenberger SC, et al. Cell Rep. 2024 Oct 22;43(10):114811. doi: 10.1016/j.celrep.2024.114811. Epub 2024 Oct 8. Cell Rep. 2024. PMID: 39383036 Free PMC article. - BCR ligation selectively inhibits IgE class switch recombination.
Wade-Vallance AK, Yang Z, Libang JB, Krishnapura AR, Jung JB, Matcham EW, Robinson MJ, Allen CDC. Wade-Vallance AK, et al. bioRxiv [Preprint]. 2024 Sep 23:2024.09.18.613749. doi: 10.1101/2024.09.18.613749. bioRxiv. 2024. PMID: 39345367 Free PMC article. Preprint. - Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances.
Zhao L, Jin S, Wang S, Zhang Z, Wang X, Chen Z, Wang X, Huang S, Zhang D, Wu H. Zhao L, et al. Signal Transduct Target Ther. 2024 Aug 28;9(1):225. doi: 10.1038/s41392-024-01947-5. Signal Transduct Target Ther. 2024. PMID: 39198425 Free PMC article. Review. - Structural Mapping of Polyclonal IgG Responses to HA After Influenza Virus Vaccination or Infection.
León AN, Rodriguez AJ, Richey ST, de la Peña AT, Wolters RM, Jackson AM, Webb K, Creech CB, Yoder S, Mudd PA, Crowe JE Jr, Han J, Ward AB. León AN, et al. bioRxiv [Preprint]. 2024 Jul 11:2024.07.08.601940. doi: 10.1101/2024.07.08.601940. bioRxiv. 2024. PMID: 39026813 Free PMC article. Preprint. - Epigenetic recording of stimulation history reveals BLIMP1-BACH2 balance in determining memory B cell fate upon recall challenge.
Shao W, Wang Y, Fang Q, Shi W, Qi H. Shao W, et al. Nat Immunol. 2024 Aug;25(8):1432-1444. doi: 10.1038/s41590-024-01900-2. Epub 2024 Jul 5. Nat Immunol. 2024. PMID: 38969872
References
- MacLennan, I.C., K.M. Toellner, A.F. Cunningham, K. Serre, D.M. Sze, E. Zuniga, M.C. Cook, and C.G. Vinuesa. 2003. Extrafollicular antibody responses. Immunol. Rev. 194:8–18. - PubMed
- Liu, Y.J., J. Zhang, P.J. Lane, E.Y. Chan, and I.C. MacLennan. 1991. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 21:2951–2962. - PubMed
- Garside, P., E. Ingulli, R.R. Merica, J.G. Johnson, R.J. Noelle, and M.K. Jenkins. 1998. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 281:96–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources