Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells - PubMed (original) (raw)
. 2006 Apr 18;103(16):6326-31.
doi: 10.1073/pnas.0601223103. Epub 2006 Apr 10.
Katharina Lötzer, Steffen Jahn, Cornelia Kramer, Markus Hildner, Ellen Bretschneider, Dörte Radke, Michael Beer, Rüdiger Vollandt, Jilly F Evans, Colin D Funk, Andreas J R Habenicht
Affiliations
- PMID: 16606835
- PMCID: PMC1458877
- DOI: 10.1073/pnas.0601223103
Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells
Barbara Uzonyi et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Cysteinyl leukotrienes (cysLT), i.e., LTC4, LTD4, and LTE4, are lipid mediators derived from the 5-lipoxygenase pathway, and the cysLT receptors cysLT1-R/cysLT2-R mediate inflammatory tissue reactions. Although endothelial cells (ECs) predominantly express cysLT2-Rs, their role in vascular biology remains to be fully understood. To delineate cysLT2-R actions, we stimulated human umbilical vein EC with LTD4 and determined early induced genes. We also compared LTD4 effects with those induced by thrombin that binds to protease-activated receptor (PAR)-1. Stringent filters yielded 37 cysLT2-R- and 34 PAR-1-up-regulated genes (>2.5-fold stimulation). Most LTD4-regulated genes were also induced by thrombin. Moreover, LTD4 plus thrombin augmented gene expression when compared with each agonist alone. Strongly induced genes were studied in detail: Early growth response (EGR) and nuclear receptor subfamily 4 group A transcription factors; E-selectin; CXC ligand 2; IL-8; a disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motif 1 (ADAMTS1); Down syndrome critical region gene 1 (DSCR1); tissue factor (TF); and cyclooxygenase 2. Transcripts peaked at approximately 60 min, were unaffected by a cysLT1-R antagonist, and were superinduced by cycloheximide. The EC phenotype was markedly altered: LTD4 induced de novo synthesis of EGR1 protein and EGR1 localized in the nucleus; LTD4 up-regulated IL-8 formation and secretion; and LTD4 raised TF protein and TF-dependent EC procoagulant activity. These data show that cysLT2-R activation results in a proinflammatory EC phenotype. Because LTD4 and thrombin are likely to be formed concomitantly in vivo, cysLT2-R and PAR-1 may cooperate to augment vascular injury.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
Early LTD4- and thrombin-regulated genes in HUVECs. HUVECs were stimulated with 100 nM LTD4 or 10 nM thrombin. (A) Heatmap of genes up-regulated >2.5-fold by LTD4; thrombin was used for comparison. (B and C) Scatterplots of LTD4- and thrombin-stimulated cells versus control; lines depict 2.5-fold change. (D) Scatterplot of LTD4- versus thrombin-stimulated cells. (E and F) Comparison of gene expression of LTD4-plus-thrombin-treated cells with cells stimulated with LTD4 or thrombin alone. Probe sets were selected as described in Supporting Text. Line separates up- from down-regulated probe sets. Columns in A indicate umbilical cord preparations; dots in scatterplots indicate means of signal intensities of four (B–D) or three (E and F) umbilical cords.
Fig. 2.
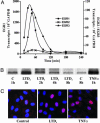
Induction of EGR transcription factor family members by LTD4. Cells were stimulated with 100 nM LTD4, and EGR QRT-PCR analyses were performed at various time points thereafter. (A) Transcript kinetics of EGR1, -2, and -3. (B) EGR1 protein is up-regulated by LTD4 or TNFα. (C) EGR1 localizes in the nucleus at 1 h.
Fig. 3.
Induction of the NR4A transcription factor family members upon LTD4 stimulation. Cells were stimulated with 100 nM LTD4, and NR4A family member transcript kinetics were performed by QRT-PCR. (Inset) NR4A1 transcript induction by 100 nM LTD4 at 1 h in the absence and presence of 10 μg/ml cycloheximide (CHX).
Fig. 4.
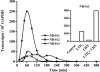
LTD4 induces IL-8 expression and secretion. Cells were stimulated with 100 nM LTD4 for the indicated time points. Supernatants were collected for ELISA, and cells were harvested for QRT-PCR analysis. (Inset) IL-8 protein content of control (C) and LTD4-stimulated (L) cultures at 2 h. Columns represent means of quadruplicate dishes ± SEM (P < 0.005; Student t test).
Fig. 5.
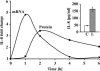
LTD4 induces TF expression and procoagulant activity. Cells were stimulated with 100 nM LTD4 for increasing periods of time. (A) TF transcript kinetics in response to LTD4 determined by QRT-PCR. (B) TF protein expression at 6 h after addition of LTD4 or TNFα. (C) TF-dependent procoagulant activity. Cells were stimulated with LTD4 for 6 h, the culture medium was removed, and TF-dependent procoagulant activity was determined in cell lysates as described in Materials and Methods. Data represent means of eight cell lysates ± SEM derived from three umbilical cords (P < 0.01; Student t test).
Similar articles
- Leukotriene C4 induces bronchoconstriction and airway vascular hyperpermeability via the cysteinyl leukotriene receptor 2 in S-hexyl glutathione-treated guinea pigs.
Yonetomi Y, Sekioka T, Kadode M, Kitamine T, Kamiya A, Matsumura N, Fujita M, Kawabata K. Yonetomi Y, et al. Eur J Pharmacol. 2015 May 5;754:98-104. doi: 10.1016/j.ejphar.2015.02.014. Epub 2015 Feb 20. Eur J Pharmacol. 2015. PMID: 25704617 - Cysteinyl leukotriene receptors.
Evans JF. Evans JF. Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:587-97. doi: 10.1016/s0090-6980(02)00057-6. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432945 Review. - ERK/Egr-1 signaling pathway is involved in CysLT2 receptor-mediated IL-8 production in HEK293 cells.
Lin K, Fang S, Cai B, Huang X, Zhang X, Lu Y, Zhang W, Wei E. Lin K, et al. Eur J Cell Biol. 2014 Jul;93(7):278-88. doi: 10.1016/j.ejcb.2014.05.001. Epub 2014 May 20. Eur J Cell Biol. 2014. PMID: 24925646 - Functional characterisation of receptors for cysteinyl leukotrienes in smooth muscle.
Jonsson EW. Jonsson EW. Acta Physiol Scand Suppl. 1998 Mar;641:1-55. Acta Physiol Scand Suppl. 1998. PMID: 9597121 Review.
Cited by
- Montelukast and Acute Coronary Syndrome: The Endowed Drug.
Alomair BM, Al-Kuraishy HM, Al-Gareeb AI, Al-Hamash SM, De Waard M, Sabatier JM, Saad HM, El-Saber Batiha G. Alomair BM, et al. Pharmaceuticals (Basel). 2022 Sep 14;15(9):1147. doi: 10.3390/ph15091147. Pharmaceuticals (Basel). 2022. PMID: 36145367 Free PMC article. Review. - Cysteinyl leukotriene 2 receptor promotes endothelial permeability, tumor angiogenesis, and metastasis.
Duah E, Teegala LR, Kondeti V, Adapala RK, Keshamouni VG, Kanaoka Y, Austen KF, Thodeti CK, Paruchuri S. Duah E, et al. Proc Natl Acad Sci U S A. 2019 Jan 2;116(1):199-204. doi: 10.1073/pnas.1817325115. Epub 2018 Dec 17. Proc Natl Acad Sci U S A. 2019. PMID: 30559191 Free PMC article. - Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression.
Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Shelton RC, et al. Mol Psychiatry. 2011 Jul;16(7):751-62. doi: 10.1038/mp.2010.52. Epub 2010 May 18. Mol Psychiatry. 2011. PMID: 20479761 Free PMC article. - Modelling negative feedback networks for activating transcription factor 3 predicts a dominant role for miRNAs in immediate early gene regulation.
Tindall MJ, Clerk A. Tindall MJ, et al. PLoS Comput Biol. 2014 May 8;10(5):e1003597. doi: 10.1371/journal.pcbi.1003597. eCollection 2014 May. PLoS Comput Biol. 2014. PMID: 24811474 Free PMC article. - RNA-seq reveals novel transcriptome of genes and their isoforms in human pulmonary microvascular endothelial cells treated with thrombin.
Zhang LQ, Cheranova D, Gibson M, Ding S, Heruth DP, Fang D, Ye SQ. Zhang LQ, et al. PLoS One. 2012;7(2):e31229. doi: 10.1371/journal.pone.0031229. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359579 Free PMC article.
References
- Funk C. D. Science. 2001;294:1871–1875. - PubMed
- Samuelsson B. Science. 1983;220:568–575. - PubMed
- Maclouf J., Murphy R. C., Henson P. M. Blood. 1989;74:703–707. - PubMed
- Sala A., Testa T., Folco G. FEBS Lett. 1996;388:94–98. - PubMed
- Brink C., Dahlen S. E., Drazen J., Evans J. F., Hay D. W., Nicosia S., Serhan C. N., Shimizu T., Yokomizo T. Pharmacol. Rev. 2003;55:195–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous