Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix - PubMed (original) (raw)
Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix
Yana K Reshetnyak et al. Proc Natl Acad Sci U S A. 2006.
Abstract
We have previously observed the spontaneous, pH-dependent insertion of a water-soluble peptide to form a helix across lipid bilayers [Hunt, J. F., Rath, P., Rothschild, K. J. & Engelman, D. M. (1997) Biochemistry 36, 15177-15192]. We now use a related peptide, pH (low) insertion peptide, to translocate cargo molecules attached to its C terminus across the plasma membranes of living cells. Translocation is selective for low pH, and various types of cargo molecules attached by disulfides can be released by reduction in the cytoplasm, including peptide nucleic acids, a cyclic peptide (phalloidin), and organic compounds. Because a high extracellular acidity is characteristic of a variety of pathological conditions (such as tumors, infarcts, stroke-afflicted tissue, atherosclerotic lesions, sites of inflammation or infection, or damaged tissue resulting from trauma) or might be created artificially, pH (low) insertion peptide may prove a useful tool for selective delivery of agents for drug therapy, diagnostic imaging, genetic control, or cell regulation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
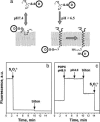
Fig. 1.
pHLIP insertion and topology. (a) Schematic diagram of cargo molecule delivery into a cell. At physiological pH, the peptide–cargo conjugate interacts weakly with a membrane. At low pH, the peptide forms a transmembrane helix with its C terminus inserted in the cytoplasm. Reduction of the disulfide bond releases a drug. (b) The topology of the pHLIP peptide in a lipid bilayer was determined by using the NBD–dithionite quenching reaction. The fluorescence signal of NBD attached to the N terminus of peptide was monitored at 530 nm when excited at 470 nm. Sodium dithionite was added to the NBD peptide inserted into POPC large unilamellar vesicles. (c) POPC large unilamellar vesicles containing dithionite ion inside were added to the NBD peptide at pH 8.0, and then decreasing the pH triggered insertion of the peptide in liposomes. Triton X-100 was used for the disruption of liposomes. The concentration of the peptide used in the experiments was 7 μM.
Fig. 2.
pHLIP transport of dansyl into cells. (a) Fluorescence images of HeLa cells incubated (for 15 min) with cleavable pHLIP–S–S–dansyl construct (7 μM) at pH 5.5, 6.5, 7.0, and 7.4 and washed with PBS buffer at pH 7.4 are shown. (b) Quantification of fluorescence images. The fluorescence signal of cells at pH 5.5 was taken as 100%. The uptake of dansyl strongly decreases with an increase of extracellular incubation pH.
Fig. 3.
The delivery of PNA into cells by pHLIP. (a) Fluorescence and phase-contrast images of HeLa cells incubated (for 30 min) with a pHLIP–S–S–PNA–TAMRA cleavable construct (1 μM) at pH 7.4 (Left) and 6.5 (Right) are shown. No translocation was observed of pHLIP–S–S–PNA–TAMRA at pH 7.4 or PNA–TAMRA at pH 7.4 or 6.5 (data not shown). (b) HeLa cells labeled with PNA–TAMRA translocated by pHLIP at pH 6.5 (Left) and the same cells treated with SYTOX–Green (Right). The majority of cells have only PNA–TAMRA, whereas only one cell had both PNA–TAMRA and SYTOX–Green.
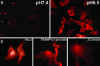
Fig. 4.
The delivery of phalloidin into cells by pHLIP. (a) Fluorescence images of HeLa cells incubated (for 1 h) with a pHLIP–S–S–Ph–TRITC cleavable construct (2 μM) at pH 7.4 (Left) and 6.5 (Right) are shown. The fluorescence was extremely weak after pH 7.4 incubation and localized to the plasma membrane. Strong fluorescence of actin filaments was observed after pH 6.5 incubation. (b) Images of HeLa (Left), breast JC (Center), and prostate TRAMP-C1 (Right) cancer cells with fluorescent actin filaments are shown. Cells were incubated for 1 h with the cleavable pHLIP–S–S–Ph–TRITC (0.5–1 μM) at pH 6.5 followed by washing with PBS at pH 7.4.
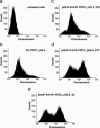
Fig. 5.
Cytofluorometry of HeLa cells. (a and b) The untreated cells (a) and cells treated with Ph–TRITC (b) at pH 6.5 and 37°C are shown. (c_–_e) Cells treated (for 1 h) with 6 μM pHLIP–S–S–Ph–TRITC at pH 7.4 and 37°C (c), pH 6.5 and 37°C (d), and pH 6.5 and 4°C (e) are shown.
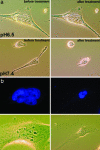
Fig. 6.
Cell phenotypes induced by phalodin transport. (a) Phase-contrast images of HeLa cells incubated (for 1 h) with pHLIP–S–S–Ph–TRITC (1 μM) at pH 6.5 and 7.4 followed by washing with PBS (pH 7.4) before (Left) and 5 min after adding of the dissociation solution (Right). Cells treated with the peptide–phalloidin at low pH remained unchanged, consistent with stabilization of the cytoskeleton by Ph–TRITC delivered by the pHLIP. (b) Fluorescence images of nuclei stained with DAPI (0.5 μM) and corresponding phase-contrast images of the multinucleated HeLa cells are presented. Multinucleation was observed at 48 h after treatment of cells with of pHLIP–S–S–Ph–TRITC (1 μM) at pH 6.5 for 1 h.
Similar articles
- Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane.
Reshetnyak YK, Andreev OA, Segala M, Markin VS, Engelman DM. Reshetnyak YK, et al. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15340-5. doi: 10.1073/pnas.0804746105. Epub 2008 Sep 30. Proc Natl Acad Sci U S A. 2008. PMID: 18829441 Free PMC article. - Tumor-Targeted, Cytoplasmic Delivery of Large, Polar Molecules Using a pH-Low Insertion Peptide.
Svoronos AA, Bahal R, Pereira MC, Barrera FN, Deacon JC, Bosenberg M, DiMaio D, Glazer PM, Engelman DM. Svoronos AA, et al. Mol Pharm. 2020 Feb 3;17(2):461-471. doi: 10.1021/acs.molpharmaceut.9b00883. Epub 2020 Jan 13. Mol Pharm. 2020. PMID: 31855437 Free PMC article. - Modulation of the pHLIP transmembrane helix insertion pathway.
Karabadzhak AG, Weerakkody D, Wijesinghe D, Thakur MS, Engelman DM, Andreev OA, Markin VS, Reshetnyak YK. Karabadzhak AG, et al. Biophys J. 2012 Apr 18;102(8):1846-55. doi: 10.1016/j.bpj.2012.03.021. Biophys J. 2012. PMID: 22768940 Free PMC article. - pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents.
Andreev OA, Engelman DM, Reshetnyak YK. Andreev OA, et al. Mol Membr Biol. 2010 Oct;27(7):341-52. doi: 10.3109/09687688.2010.509285. Epub 2010 Oct 13. Mol Membr Biol. 2010. PMID: 20939768 Free PMC article. Review. - Transmembrane helices before, during, and after insertion.
White SH, von Heijne G. White SH, et al. Curr Opin Struct Biol. 2005 Aug;15(4):378-86. doi: 10.1016/j.sbi.2005.07.004. Curr Opin Struct Biol. 2005. PMID: 16043344 Review.
Cited by
- pHLIP peptide targets nanogold particles to tumors.
Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA. Yao L, et al. Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):465-70. doi: 10.1073/pnas.1219665110. Epub 2012 Dec 24. Proc Natl Acad Sci U S A. 2013. PMID: 23267062 Free PMC article. - Ions at the Interface: Pushing the pK of pHLIP.
Schlebach JP. Schlebach JP. Biophys J. 2019 Sep 3;117(5):793-794. doi: 10.1016/j.bpj.2019.07.035. Epub 2019 Jul 29. Biophys J. 2019. PMID: 31400915 Free PMC article. No abstract available. - Peptides of pHLIP family for targeted intracellular and extracellular delivery of cargo molecules to tumors.
Wyatt LC, Moshnikova A, Crawford T, Engelman DM, Andreev OA, Reshetnyak YK. Wyatt LC, et al. Proc Natl Acad Sci U S A. 2018 Mar 20;115(12):E2811-E2818. doi: 10.1073/pnas.1715350115. Epub 2018 Mar 5. Proc Natl Acad Sci U S A. 2018. PMID: 29507241 Free PMC article. - The bovine papillomavirus E5 protein and the PDGF beta receptor: it takes two to tango.
Talbert-Slagle K, DiMaio D. Talbert-Slagle K, et al. Virology. 2009 Feb 20;384(2):345-51. doi: 10.1016/j.virol.2008.09.033. Epub 2008 Nov 6. Virology. 2009. PMID: 18990418 Free PMC article. Review. - Stimuli-responsive nanoparticles for targeting the tumor microenvironment.
Du J, Lane LA, Nie S. Du J, et al. J Control Release. 2015 Dec 10;219:205-214. doi: 10.1016/j.jconrel.2015.08.050. Epub 2015 Sep 1. J Control Release. 2015. PMID: 26341694 Free PMC article. Review.
References
- Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. J. Mol. Biol. 2001;305:567–580. - PubMed
- Lehnert U., Xia Y., Royce T. E., Goh C. S., Liu Y., Senes A., Yu H., Zhang Z. L., Engelman D. M., Gerstein M. Q. Rev. Biophys. 2004;37:121–146. - PubMed
- Popot J.-L., Engelman D. M. Biochemistry. 1990;29:4031–4037. - PubMed
- Engelman D. M., Chen Y., Chin C. N., Curran A. R., Dixon A. M., Dupuy A. D., Lee A. S., Lehnert U., Matthews E. E., Reshetnyak Y. K., et al. FEBS Lett. 2003;555:122–125. - PubMed
- Van den Berg B., Clemons W. M., Jr., Collinson I., Modis Y., Hartmann E., Harrison S. C., Rapoport T. A. Nature. 2004;427:36–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources