Cell Life versus cell longevity: the mysteries surrounding the NAD+ precursor nicotinamide - PubMed (original) (raw)
Review
Cell Life versus cell longevity: the mysteries surrounding the NAD+ precursor nicotinamide
Faqi Li et al. Curr Med Chem. 2006.
Abstract
Nicotinamide, the amide form of niacin (vitamin B(3)), is the precursor for the coenzyme beta-nicotinamide adenine dinucleotide (NAD(+)) and plays a significant role during the enhancement of cell survival as well as cell longevity. Yet, these abilities of nicotinamide appear to be diametrically opposed. Here we describe the development of nicotinamide as a novel agent that is critical for modulating cellular metabolism, plasticity, longevity, and inflammatory microglial function as well as for influencing cellular life span. The capacity of nicotinamide to govern not only intrinsic cellular integrity, but also extrinsic cellular inflammation rests with the modulation of a host of cellular targets that involve mitochondrial membrane potential, poly(ADP-ribose) polymerase, protein kinase B (Akt), Forkhead transcription factors, Bad, caspases, and microglial activation. Further knowledge acquired in regards to the ability of nicotinamide to foster cellular survival and regulate cellular lifespan should significantly promote the development of therapies against a host of disorders, such as aging, Alzheimer's disease, diabetes, cerebral ischemia, Parkinson's disease, and cancer.
Figures
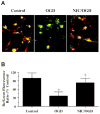
Fig. 1
Nicotinamide (NIC) prevents the loss of mitochondrial membrane potential during oxygen glucose deprivation (OGD). (A) Representative pictures demonstrate that exposure to a 3 hour period of OGD produced a significant decrease in the red/green fluorescence intensity ratio in cultured rat hippocampal neurons using a cationic membrane potential indicator JC-1 within 3 hours when compared with untreated control cultures, suggesting that OGD results in mitochondrial membrane depolarization. Application of NIC (12.5 mM) 1 hour prior to OGD exposure significantly increased the red/green fluorescence intensity of neurons, indicating that mitochondrial membrane potential was restored. (B) The relative ratio of red/green fluorescent intensity of mitochondrial staining in both untreated (control) neurons and neurons exposed to OGD or NIC (12.5 mM) plus OGD 3 hour following the initial insult was measured in 4 independent experiments with analysis performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at
http://rsb.info.nih.gov/nih-image/
) (Control vs. OGD, *p_<0.01; OGD vs. NIC/OGD, †p<_0.01).
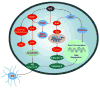
Fig. 2
Nicotinamide (NIC) modulates a variety of cellular mediators to oversee cellular metabolism, longevity, survival, and inflammatory microglial activation. Nicotinamide promotes cellular function and survival through a series of pathways that involve NAD+, cell senescence mechanisms, the serine-threonine kinase Akt and its downstream substrates of FOXO3a, Bad, and caspases. Closely to the cytoprotection by nicotinamide is the maintenance of mitochondrial membrane potential, mitochondrial energy reserves, cytochrome c (Cyto c) release, and PARP. Targeting by NIC of specific caspase pathways ultimately serves to preserve genomic integrity and prevent early apoptotic injury “tagging” for microglial disposal. NAD: β-nicotinamide adenine dinucleotide; Sir2: Silent information regulator 2; MG: microglia; Mito: mitochondria; PS: phosphatidylserine.
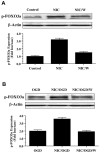
Fig. 3
Nicotinamide (NIC) increases phosphorylation of p-FOXO3a through activation of Akt during oxidative stress. (A) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted at 6 hours following NIC (12.5 mM) treatment with anti - phospho-FOXO3a (p- FOXO3a) antibody. NIC increased the expression of p-FOXO3a significantly over a 6 hour period. Co-application of NIC with phosphoinositol-3 kinase inhibitor wortmannin (W, 500 μM), which inhibits phosphorylation of Akt, decreased p-FOXO3a expression during NIC application. (B) Equal amounts of neuronal protein extracts (50 μg/lane) were immunoblotted at 6 hours following oxygen glucose deprivation (OGD), NIC (12.5 mM), or NIC (12.5 mM) following a 3 hour period of OGD with anti - phospho-FOXO3a (p- FOXO3a) antibody. OGD alone increased the expression of p-FOXO3a. The expression of p-FOXO3a was further increased in neurons with NIC applied 1 hour prior to OGD. Yet, application of wortmannin (W) significantly decreased the expression of p-FOXO3a during the administration of NIC during OGD.
Similar articles
- Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide.
Li F, Chong ZZ, Maiese K. Li F, et al. Front Biosci. 2004 Sep 1;9:2500-20. doi: 10.2741/1412. Front Biosci. 2004. PMID: 15353303 Review. - Protective effect of nicotinamide against poly(ADP-ribose) polymerase-1-mediated astrocyte death depends on its transporter-mediated uptake.
Suzuki E, Okuda H, Nishida K, Fujimoto S, Nagasawa K. Suzuki E, et al. Life Sci. 2010 Apr 24;86(17-18):676-82. doi: 10.1016/j.lfs.2010.02.019. Epub 2010 Feb 25. Life Sci. 2010. PMID: 20188745 - NAD+ and vitamin B3: from metabolism to therapies.
Sauve AA. Sauve AA. J Pharmacol Exp Ther. 2008 Mar;324(3):883-93. doi: 10.1124/jpet.107.120758. Epub 2007 Dec 28. J Pharmacol Exp Ther. 2008. PMID: 18165311 Review. - Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death.
Abeti R, Abramov AY, Duchen MR. Abeti R, et al. Brain. 2011 Jun;134(Pt 6):1658-72. doi: 10.1093/brain/awr104. Brain. 2011. PMID: 21616968 - The vitamin nicotinamide: translating nutrition into clinical care.
Maiese K, Chong ZZ, Hou J, Shang YC. Maiese K, et al. Molecules. 2009 Sep 9;14(9):3446-85. doi: 10.3390/molecules14093446. Molecules. 2009. PMID: 19783937 Free PMC article. Review.
Cited by
- Programming apoptosis and autophagy with novel approaches for diabetes mellitus.
Maiese K. Maiese K. Curr Neurovasc Res. 2015;12(2):173-88. doi: 10.2174/1567202612666150305110929. Curr Neurovasc Res. 2015. PMID: 25742566 Free PMC article. Review. - Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin.
Chong ZZ, Shang YC, Wang S, Maiese K. Chong ZZ, et al. Prog Neurobiol. 2012 Nov;99(2):128-48. doi: 10.1016/j.pneurobio.2012.08.001. Epub 2012 Aug 15. Prog Neurobiol. 2012. PMID: 22980037 Free PMC article. Review. - The "O" class: crafting clinical care with FoxO transcription factors.
Maiese K, Chong ZZ, Hou J, Shang YC. Maiese K, et al. Adv Exp Med Biol. 2009;665:242-60. doi: 10.1007/978-1-4419-1599-3_18. Adv Exp Med Biol. 2009. PMID: 20429429 Free PMC article. Review. - Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling.
Chong ZZ, Shang YC, Maiese K. Chong ZZ, et al. Curr Neurovasc Res. 2007 Aug;4(3):194-204. doi: 10.2174/156720207781387150. Curr Neurovasc Res. 2007. PMID: 17691973 Free PMC article. - New Insights for nicotinamide: Metabolic disease, autophagy, and mTOR.
Maiese K. Maiese K. Front Biosci (Landmark Ed). 2020 Jun 1;25(11):1925-1973. doi: 10.2741/4886. Front Biosci (Landmark Ed). 2020. PMID: 32472766 Free PMC article. Review.
References
- Li F, Chong ZZ, Maiese K. Front Biosci. 2004;9:2500–2520. - PubMed
- Maiese K, Chong ZZ. Trends Pharmacol Sci. 2003;24(5):228–32. - PubMed
- DiPalma JR, Thayer WS. Annu Rev Nutr. 1991;11:169–87. - PubMed
- Jackson TM, Rawling JM, Roebuck BD, Kirkland JB. J Nutr. 1995;125(6):1455–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES006639/ES/NIEHS NIH HHS/United States
- R01 NS053946/NS/NINDS NIH HHS/United States
- R01 NS053946-01A2/NS/NINDS NIH HHS/United States
- P30 ES06639/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials