Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders - PubMed (original) (raw)
Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders
George K Tofaris et al. J Neurosci. 2006.
Abstract
Dysfunction of the 140 aa protein alpha-synuclein plays a central role in Lewy body disorders, including Parkinson's disease, as well as in multiple system atrophy. Here, we show that the expression of truncated human alpha-synuclein(1-120), driven by the rat tyrosine hydroxylase promoter on a mouse alpha-synuclein null background, leads to the formation of pathological inclusions in the substantia nigra and olfactory bulb and to a reduction in striatal dopamine levels. At the behavioral level, the transgenic mice showed a progressive reduction in spontaneous locomotion and an increased response to amphetamine. These findings suggest that the C-terminal of alpha-synuclein is an important regulator of aggregation in vivo and will help to understand the mechanisms underlying the pathogenesis of Lewy body disorders and multiple system atrophy.
Figures
Fig. 1.
Expression of α-Syn120 in transgenic mouse brain. A, Schematic diagram of the construct: human α-synuclein(1–120) DNA was cloned downstream of the rat TH promoter. B, Immunoblot probed for α-synuclein (antibody Syn-1) and TH. The tissues were obtained from 6-week-old transgenic mice and littermate controls. Cx, Cerebral cortex; Cb, cerebellum; OB, olfactory bulb; SN, substantia nigra. No endogenous α-synuclein was detected at 19 kDa. C, Expression levels of α-Syn120 in olfactory bulb of transgenic mice compared with full-length α-synuclein in olfactory bulb from C57BL/6 mice. D, E, Immunoblotting (antibody Syn-1) of extracts from olfactory bulb (D) and substantia nigra (E) at 6, 12, and 24 months showed stable expression of the transgene. rS, Recombinant full-length human α-synuclein.
Fig. 2.
Cellular localization of α-Syn120 in the substantia nigra of transgenic mice. a–d, Adjacent coronal sections of the substantia nigra from 6-month-old transgenic (a, b) and littermate control (c, d) mice were stained for TH and α-synuclein (Syn-1). TH-positive neurons from transgenic mice (a) showed somal expression of α-Syn120 (b). A similar region from littermate control mice was positive for TH (c) but negative for α-synuclein (d), as would be expected in mice with a null α-synuclein background. e, f, Immunofluorescence microscopy of the substantia nigra using anti-TH (e, red) and anti-α-synuclein (f, green) antibodies showed colocalization (g, yellow). Scale bar: (in a) a–d, 200 μm; e–g, 250 μm.
Fig. 3.
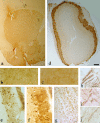
Histological characterization of α-Syn120 in the striatum and the olfactory bulb. a, Punctate neuropil localization of α-Syn120 in the striatum was consistent with enrichment at synaptic nerve terminals. b, c, TH staining showed enlarged nerve cell processes in striatum of transgenic (b) but not in littermate control (c) mice. h–j, Abnormal axonal morphology was confirmed by phosphorylated neurofilament staining with SMI 31 antibody in transgenic (h, i) compared with littermate control (j) mice. α-Syn120 immunoreactivity (syn-1 antibody) in the olfactory bulb (d) from a transgenic mouse. e, At a higher magnification, TH staining appeared diffusely distributed along the somatodendritic compartment. Abundant α-Syn120-syn-1-positive inclusions (f) were present in the olfactory bulb at all ages examined, showing a different distribution of the protein compared with endogenous full-length α-synuclein in C57BL/6 mice stained with the same antibody (g). Scale bars: a, d, 600 μm; (in h) h–j, 5 μm; (in g) b, c, e, f, 150 μm.
Fig. 4.
Histological characterization of α-Syn120 in the substantia nigra. a, At 3 months of age, α-Syn120 was diffusely distributed through the somatodendritic compartment. b, At 14 months, α-Syn120 immunoreactivity revealed the presence of pyknotic perikarya. c, e, Between 11 and 14 months, a number of pathological profiles was observed, which included beaded (c) or swollen (e) processes. Perinuclear aggregates (d and j, arrows) or dense inclusions (f, arrowheads) within TH neurons (g, arrowhead, double-stained neurons with anti-TH blue and Syn1 brown) are shown. Vacuolation of the cytoplasm (arrows h, i) was evident at this stage (h–j). The following α-synuclein antibodies were used: Syn-1, a, b, e–i; PER7, c, d; SYN h119, j. Scale bar, 60 μm.
Fig. 5.
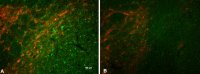
Microglial activation. Double-staining immunofluorescence with TH (red) and CD11b (green) shows increased microglial cell numbers in the SN of transgenic mice (A) compared with littermate controls (B).
Fig. 6.
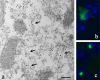
Ultrastructure of α-Syn120 aggregates. a, Immunoelectron microscopy (antibody Syn-1) of the olfactory bulb from 7-month-old transgenic mice revealed granular and filamentous deposits. Arrows point to filamentous structures. b, c, Thioflavin S fluorescence in olfactory bulb (b) and substantia nigra (c). Nuclei were visualized with DAPI (blue fluorescence). Scale bar: a, 250 nm.
Fig. 7.
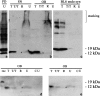
Sequential extraction of α-Syn120 from substantia nigra and olfactory bulb. Substantia nigra and olfactory bulb tissues from 6-month-old transgenic mice were sequentially extracted with Tris-HCl, Triton X-100, RIPA buffer, and urea, followed by immunoblotting with antibodies Syn-1 (a–c), Syn204 (d), and PER7 (e). a, The urea-soluble material from the substantia nigra of a PD patient was used as a positive control. In age-matched C57BL/6 wild-type mice with endogenous α-synuclein, the protein was detected only in soluble fractions (c) but not in urea extracts (U in c, CU in d, e). CU, Urea extract from control wild-type mice with endogenous α-synuclein. The asterisk indicates the position of the monomeric α-Syn120 band in the urea-soluble fractions. SN, Substantia nigra; U, urea; T, Tris-HCl; R, RIPA buffer; T/T, Triton X-100; OB, olfactory bulb.
Fig. 8.
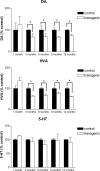
Striatal dopamine (DA), homovanillic acid (HVA), and 5-HT levels in transgenic and littermate control mice at different ages. Top panel, A significant reduction (p < 0.0001) in striatal dopamine levels was present in transgenic mice at 3, 6, 9, and 12 months of age but not in 1-month-old animals. Middle panel, This was paralleled by a similar reduction (p < 0.003) in the levels of homovanillic acid. Bottom panel, Striatal 5-HT levels did not differ significantly between control and transgenic mice. Error bars represent SEM.
Fig. 9.
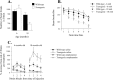
Spontaneous and amphetamine-induced activity in 6- and 18-month-old α-Syn120 transgenic and control mice. A, Eighteen-month-old transgenic mice showed reduced levels of spontaneous activity, measured as infrared beam breaks, compared with equivalent aged wild-type mice, and, overall, the older mice showed a reduction in activity relative to 6 month-old subjects. B, When examined in more detail, all of the groups of mice habituated to the environment equally during the 30 min test. After 30 min habituation, mice received injections of either saline vehicle or 1 mg/kg
d
-amphetamine intraperitoneally, counterbalanced over two sessions 7 d apart, and activity was monitored for an additional 120 min. Amphetamine induced increased activity in all mice, with a peak response 60 min after injection (n = 11 and 13 for 6-month-old, and n = 12 and 12 for 18-month-old transgenic and control mice, respectively). C, Compared with wild-type mice at both ages and 6-month-old α-Syn120 transgenic mice, 18-month-old α-Syn120 mice exhibited a significantly enhanced response to amphetamine relative to the preinjection activity performance.
## Similar articles
* Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson's disease model. Wegrzynowicz M, Bar-On D, Calo' L, Anichtchik O, Iovino M, Xia J, Ryazanov S, Leonov A, Giese A, Dalley JW, Griesinger C, Ashery U, Spillantini MG. Wegrzynowicz M, et al. Acta Neuropathol. 2019 Oct;138(4):575-595. doi: 10.1007/s00401-019-02023-x. Epub 2019 May 31. Acta Neuropathol. 2019. PMID: 31165254 Free PMC article. * Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston WJ, McGowan E, Farrer M, Hardy J, Duff K, Przedborski S, Di Monte DA. Matsuoka Y, et al. Neurobiol Dis. 2001 Jun;8(3):535-9. doi: 10.1006/nbdi.2001.0392. Neurobiol Dis. 2001. PMID: 11442360 * AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease. Ip CW, Klaus LC, Karikari AA, Visanji NP, Brotchie JM, Lang AE, Volkmann J, Koprich JB. Ip CW, et al. Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article. * The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Spillantini MG, Goedert M. Spillantini MG, et al. Ann N Y Acad Sci. 2000;920:16-27. doi: 10.1111/j.1749-6632.2000.tb06900.x. Ann N Y Acad Sci. 2000. PMID: 11193145 Review. * Proteomic approach to studying Parkinson's disease. Zhang J, Goodlett DR. Zhang J, et al. Mol Neurobiol. 2004 Jun;29(3):271-88. doi: 10.1385/MN:29:3:271. Mol Neurobiol. 2004. PMID: 15181239 Review.
## Cited by
* Interaction between Neuromelanin and Alpha-Synuclein in Parkinson's Disease. Xu S, Chan P. Xu S, et al. Biomolecules. 2015 Jun 5;5(2):1122-42. doi: 10.3390/biom5021122. Biomolecules. 2015. PMID: 26057626 Free PMC article. Review. * Immune system responses in Parkinson's disease: Early and dynamic. Tansey MG, Romero-Ramos M. Tansey MG, et al. Eur J Neurosci. 2019 Feb;49(3):364-383. doi: 10.1111/ejn.14290. Epub 2018 Dec 10. Eur J Neurosci. 2019. PMID: 30474172 Free PMC article. Review. * The physiological role of α-synuclein and its relationship to Parkinson's Disease. Sulzer D, Edwards RH. Sulzer D, et al. J Neurochem. 2019 Sep;150(5):475-486. doi: 10.1111/jnc.14810. Epub 2019 Jul 28. J Neurochem. 2019. PMID: 31269263 Free PMC article. Review. * Modulation of alpha-synuclein expression in transgenic animals for modelling synucleinopathies--is the juice worth the squeeze? Buchman VL, Ninkina N. Buchman VL, et al. Neurotox Res. 2008 Dec;14(4):329-41. doi: 10.1007/BF03033857. Neurotox Res. 2008. PMID: 19073436 Free PMC article. Review. * Low-Expressing Synucleinopathy Mouse Models Based on Oligomer-Forming Mutations and C-Terminal Truncation of α-Synuclein. Martinez Hernandez A, Silbern I, Geffers I, Tatenhorst L, Becker S, Urlaub H, Zweckstetter M, Griesinger C, Eichele G. Martinez Hernandez A, et al. Front Neurosci. 2021 Jun 17;15:643391. doi: 10.3389/fnins.2021.643391. eCollection 2021. Front Neurosci. 2021. PMID: 34220415 Free PMC article.
## References
1. 1. Antony T, Hoyer W, Cherny D, Heim G, Jovin TM, Subramaniam V (2003). Cellular polyamines promote the aggregation of α-synuclein. J Biol Chem 278:3235–3240. - PubMed 2. 1. Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T (1998). Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152:879–884. - PMC - PubMed 3. 1. Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. - PubMed 4. 1. Cai G, Wang HY, Friedman E (2002). Increased dopamine receptor signaling and dopamine receptor-G protein coupling in denervated striatum. J Pharmacol Exp Ther 302:1105–1112. - PubMed 5. 1. Campbell BCV, Li QL, Culvenor JG, Jäkälä P, Cappai R, Beyreuther K, Masters CL, McLean CA (2000). Accumulation of insoluble α-synuclein in dementia with Lewy bodies. Neurobiol Dis 7:192–200. - PubMed
## Publication types
## MeSH terms
## Substances
## LinkOut - more resources
* ### Full Text Sources * Europe PubMed Central * HighWire * PubMed Central * ### Other Literature Sources * The Lens - Patent Citations Database * ### Medical * MedlinePlus Consumer Health Information * MedlinePlus Health Information * ### Molecular Biology Databases * Mouse Genome Informatics (MGI)