Tubulin-mediated binding of human immunodeficiency virus-1 Tat to the cytoskeleton causes proteasomal-dependent degradation of microtubule-associated protein 2 and neuronal damage - PubMed (original) (raw)
Tubulin-mediated binding of human immunodeficiency virus-1 Tat to the cytoskeleton causes proteasomal-dependent degradation of microtubule-associated protein 2 and neuronal damage
Susanna Aprea et al. J Neurosci. 2006.
Abstract
One of the hallmarks of human immunodeficiency virus (HIV)-1 associated pathology in the CNS is deterioration of neuronal processes. Although there is mounting evidence of neuronal toxicity and cell death induced by the HIV-1 transactivating factor Tat, the molecular events linked directly to its detrimental effect on neuronal cells remain unclear. In this study, we used rat embryonic cortical neurons and demonstrated that Tat causes rapid degradation of microtubule-associated protein 2 (MAP2) and the collapse of cytoskeletal filaments. The mechanism of Tat action on MAP2 stability involved Tat-mediated translocation of the proteasome to the site of microtubule filaments. Immunohistochemical analysis of clinical samples from patients with HIV encephalopathy further revealed a significant decrease in MAP2 with predominant cytoplasmic 20S in cortical neurons near microglial nodules. These findings indicate a novel mechanism for the action of Tat on neuronal cells. It involves proteasome-mediated MAP2 degradation and may account for the loss of MAP2 and neuronal damage observed in the brain of AIDS patients with neurological dysfunctions.
Figures
Figure 1.
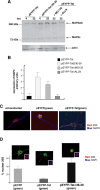
HIV-1 Tat expression and its damaging effects on rat embryonic cortical neurons. A, Representative Western blot showing levels of expression of fusion protein EYFP-Tat and control protein EYFP in rat cortical neurons at the indicated time points after nucleofection (see Materials and Methods). EYFP and EYFP-Tat proteins were detected using an antibody that recognizes YFP (top panel). The same blot was probed with anti-Tat antibody (middle panel) and with anti-actin antibody to verify equal loading (bottom panel). B, Expression of EYFP and EYFP-Tat detected by fluorescent light microscopy at 4 and 24 h (hrs) after nucleofection (original magnification, 100×). C, Fluorescent images showing differentiated neurons cotransfected either with pECFP (in blue) and the mitochondrial-targeted dsRed or with pEFP-Tat and dsRed-mito at 32 h after nucleofection (original magnification, 400×). The images were taken using an inverted fluorescent microscope equipped with motorized _z_-axis and deconvolution software. D, Representative pictures showing expression of EYFP and EYFP-Tat proteins in mature neurons at 4 and 24 h after transfection (original magnification, 200×).
Figure 2.
Tat-induced MAP2 degradation. A, Western blot analysis of protein extracts obtained from cultured rat cortical neurons at the indicated time points. Anti-MAP2 antibody recognizes MAP2 isoforms at 300 kDa (MAP2a/b) and 75 kDa (MAP2c). B, Immunofluorescence was performed using anti-MAP2 antibody (red) in pEYFP and pEYFP-Tat expressing neurons at 4 and 24 h (hrs). Colocalization of EYFP and MAP2 is indicated by yellow fluorescence. Tat-positive cells are indicated by green fluorescence (original magnification, 400×). C, Deconvolution evaluation of EYFP and EYFP-Tat cells fixed 24 h after transfection and immunolabeled with anti-MAP2 antibody. Note that Tat-expressing cell is MAP2 negative and is also characterized by loss of nuclear staining (DAPI) (original magnification, 400×). D, Western blot analysis showing expression of MAP2a/b isoforms in EYFP-Tat cells and pEYFP-positive cells at 4 and 24 h time points. Anti-Grb2 antibody was used to confirm equal loading. E, Densitometric analysis of MAP2a/b expression in EYFP and EYFP-Tat-positive cells normalized by Grb2 and by the efficiency of transfection. Values represent the average of three independent experiments. F, Western blot detecting intracellular Tat in control untreated neurons (lane 1, top panel) and in neurons exposed for 24 h to recombinant Tat1–72 protein at concentrations of 100 n
m
(lane 2) or 500 n
m
(lane 3). The same blot was reprobed with anti-MAP2 (middle panel) and anti-Grb2 (bottom panel) antibodies. G, Densitometric analysis relative to MAP2a/b expression after treatment with Tat1–72 evaluated from three independent experiments and normalization for Grb2 levels. Error bars represent SEM.
Figure 3.
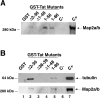
Pull-down reactions between GST-Tat fusion proteins and protein extracts isolated from differentiated cortical neurons. The Tat mutants were: TatΔ2–36 missing the amino acids 2–35; TatΔ1–48 lacks the first 48 amino acids, and Tat1–56 lacks amino acids 57–86. A, The results of the pull-downs are shown as a Western blot developed with anti MAP2a/b antibody. B, An additional GST-Tat pull-down using a mutant in which the tubulin-binding domain was deleted (Δ36–39). The same blot was probed with anti-MAP2a/b antibody (bottom panel).
Figure 4.
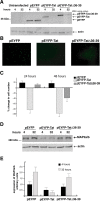
The tubulin-binding domain of Tat mediates MAP2a/b degradation. A, Western blots showing protein levels of EYFP, EYFP-Tat, and EYFP-Tat Δ36–39. The blot was developed with anti-living colors antibody that recognizes YFP. Actin was used to verify equal loading. B, Fluorescent images of EYFP, EYFP-Tat, and EYFP-TatΔ36–39-positive cells 24 h after transfection (original magnification, 100×). C, Quantitative analysis of the results from B at 24 and 48 h after transfection. Data represent the average of five independent experiments in triplicate (n = 15) with SD. D, Western blot analysis showing MAP2a/b protein level in cortical neurons at 4 and 24 h after transfection with pEYFP, pEYFP-Tat, and pEYFP-TatΔ36–39 expression vectors. E, Densitometry performed on three different Western blots obtained from independent experiments showing expression of MAP2a/b in the indicated samples. Error bars represent SEM.
Figure 5.
Tat-mediated recruitment of 20S particles and proteasomal degradation of MAP2a/b. A, Western blot of MAP2 expression in neurons transfected with pEYFP-Tat or pEYFP-TatΔ36–39 vectors. EYFP-Tat expressing cells were cultured in the presence or absence of protease inhibitors MG-132 (25 n
m
) or ALLN (0.25 μ
m
) for the indicated time points. MG-132 as well as TatΔ36–39 preserves the stability of MAP2a/b. B, Densitometric analysis of MAP2a/b expression from three independent experiments. C, Immunofluorescent detection of the 20S proteasome (red) in differentiated neurons (cultured for 5 d, untransfected) and in EYFP or EYFP-Tat neurons 24 h after transfection (original magnification, 1000×). D, Diagram showing quantification of nuclear 20S in the indicated cell types as determined by deconvolution software (original magnification, 1000×). Representative images of neurons transfected with pEYFP, pEYFP-Tat, or pEYFP-TatΔ36–39 vectors are shown above each graph. The insets in the pictures represent the area in which colocalization between 20S (red) and DAPI (blue) was calculated. Error bars represent SEM.
Figure 6.
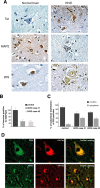
Detection of Tat, MAP2, and 20S in HIVE and control brains. A, Immunohistochemical analysis of cortical sections from a normal brain and one case of HIVE (see Materials and Methods). Black arrows indicate neurons; arrowheads indicate astrocytes (original magnification, 400×). B, Quantitative analysis showing percentage of neurons positive for MAP2 in the normal brain and in two cases of HIVE. C, Graph showing percentage of nuclear versus cytoplasmic distribution of 20S particles in normal brain and two cases of HIVE. D, Immunofluorescence analysis of HIVE brain tissues showing colocalization between 20S and Tat (top row; original magnification, 1000×) or MAP2 and Tat (bottom row; original magnification, 400×). Error bars represent SEM.
Similar articles
- Acute exposure to ethanol potentiates human immunodeficiency virus type 1 Tat-induced Ca(2+) overload and neuronal death in cultured rat cortical neurons.
Brailoiu E, Brailoiu GC, Mameli G, Dolei A, Sawaya BE, Dun NJ. Brailoiu E, et al. J Neurovirol. 2006 Feb;12(1):17-24. doi: 10.1080/13550280500516427. J Neurovirol. 2006. PMID: 16595370 - Identification of a binding site of the human immunodeficiency virus envelope protein gp120 to neuronal-specific tubulin.
Avdoshina V, Taraballi F, Dedoni S, Corbo C, Paige M, Saygideğer Kont Y, Üren A, Tasciotti E, Mocchetti I. Avdoshina V, et al. J Neurochem. 2016 Apr;137(2):287-98. doi: 10.1111/jnc.13557. Epub 2016 Mar 15. J Neurochem. 2016. PMID: 26826352 Free PMC article. - Human Immunodeficiency Virus Type 1 gp120 and Tat Induce Mitochondrial Fragmentation and Incomplete Mitophagy in Human Neurons.
Teodorof-Diedrich C, Spector SA. Teodorof-Diedrich C, et al. J Virol. 2018 Oct 29;92(22):e00993-18. doi: 10.1128/JVI.00993-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30158296 Free PMC article. - Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function.
Sánchez C, Díaz-Nido J, Avila J. Sánchez C, et al. Prog Neurobiol. 2000 Jun;61(2):133-68. doi: 10.1016/s0301-0082(99)00046-5. Prog Neurobiol. 2000. PMID: 10704996 Review. - Interactions of human immunodeficiency virus-1 proteins with neurons: possible role in the development of human immunodeficiency virus-1-associated dementia.
van de Bovenkamp M, Nottet HS, Pereira CF. van de Bovenkamp M, et al. Eur J Clin Invest. 2002 Aug;32(8):619-27. doi: 10.1046/j.1365-2362.2002.01029.x. Eur J Clin Invest. 2002. PMID: 12190962 Review.
Cited by
- HIV-1 Tat mediates degradation of RON receptor tyrosine kinase, a regulator of inflammation.
Kalantari P, Harandi OF, Hankey PA, Henderson AJ. Kalantari P, et al. J Immunol. 2008 Jul 15;181(2):1548-55. doi: 10.4049/jimmunol.181.2.1548. J Immunol. 2008. PMID: 18606710 Free PMC article. - Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways.
Del Guerra FB, Fonseca JL, Figueiredo VM, Ziff EB, Konkiewitz EC. Del Guerra FB, et al. J Neurovirol. 2013 Aug;19(4):314-27. doi: 10.1007/s13365-013-0177-7. Epub 2013 Jul 19. J Neurovirol. 2013. PMID: 23868513 Review. - Highly dynamic microtubules improve the effectiveness of early stages of human influenza A/NWS/33 virus infection in LLC-MK2 cells.
De Conto F, Di Lonardo E, Arcangeletti MC, Chezzi C, Medici MC, Calderaro A. De Conto F, et al. PLoS One. 2012;7(7):e41207. doi: 10.1371/journal.pone.0041207. Epub 2012 Jul 20. PLoS One. 2012. PMID: 22911759 Free PMC article. - Clathrin-nanoparticles deliver BDNF to hippocampus and enhance neurogenesis, synaptogenesis and cognition in HIV/neuroAIDS mouse model.
Vitaliano GD, Kim JK, Kaufman MJ, Adam CW, Zeballos G, Shanmugavadivu A, Subburaju S, McLaughlin JP, Lukas SE, Vitaliano F. Vitaliano GD, et al. Commun Biol. 2022 Mar 17;5(1):236. doi: 10.1038/s42003-022-03177-3. Commun Biol. 2022. PMID: 35301411 Free PMC article. - Mitochondria and viruses.
Ohta A, Nishiyama Y. Ohta A, et al. Mitochondrion. 2011 Jan;11(1):1-12. doi: 10.1016/j.mito.2010.08.006. Epub 2010 Sep 8. Mitochondrion. 2011. PMID: 20813204 Free PMC article. Review.
References
- Adori C, Low P, Moszkovkin G, Bagdy G, Laszlo L, Kovacs GG (2005). Subcellular distribution of components of the ubiquitin-proteasome-system in non-diseased human and rat brain. J Histochem Cytochem 54:263–267. - PubMed
- Aoki M, Abe K, Yoshida T, Hattori A, Kogure K, Itoyama Y (1995). Early immunohistochemical changes of microtubule based motor proteins in gerbil hippocampus after transient ischemia. Brain Res 669:189–196. - PubMed
- Apcher GS, Heink S, Zantopf D, Kloetzel PM, Schmid HP, Mayer RJ, Kruger E (2003). Human immunodeficiency virus-1 Tat protein interacts with distinct proteasomal alpha and beta subunits. FEBS Lett 553:200–204. - PubMed
- Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL (2004). Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol 61:369–376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials