Regulated cleavages at the West Nile virus NS4A-2K-NS4B junctions play a major role in rearranging cytoplasmic membranes and Golgi trafficking of the NS4A protein - PubMed (original) (raw)
Regulated cleavages at the West Nile virus NS4A-2K-NS4B junctions play a major role in rearranging cytoplasmic membranes and Golgi trafficking of the NS4A protein
Jojanneke Roosendaal et al. J Virol. 2006 May.
Abstract
A common feature associated with the replication of most RNA viruses is the formation of a unique membrane environment encapsulating the viral replication complex. For their part, flaviviruses are no exception, whereupon infection causes a dramatic rearrangement and induction of unique membrane structures within the cytoplasm of infected cells. These virus-induced membranes, termed paracrystalline arrays, convoluted membranes, and vesicle packets, all appear to have specific functions during replication and are derived from different organelles within the host cell. The aim of this study was to identify which protein(s) specified by the Australian strain of West Nile virus, Kunjin virus (KUNV), are responsible for the dramatic membrane alterations observed during infection. Thus, we have shown using immunolabeling of ultrathin cryosections of transfected cells that expression of the KUNV polyprotein intermediates NS4A-4B and NS2B-3-4A, as well as that of individual NS4A proteins with and without the C-terminal transmembrane domain 2K, resulted in different degrees of rearrangement of cytoplasmic membranes. The formation of the membrane structures characteristic for virus infection required coexpression of an NS4A-NS4B cassette with the viral protease NS2B-3pro which was shown to be essential for the release of the individual NS4A and NS4B proteins. Individual expression of NS4A protein retaining the C-terminal transmembrane domain 2K resulted in the induction of membrane rearrangements most resembling virus-induced structures, while removal of the 2K domain led to a less profound membrane rearrangement but resulted in the redistribution of the NS4A protein to the Golgi apparatus. The results show that cleavage of the KUNV polyprotein NS4A-4B by the viral protease is the key initiation event in the induction of membrane rearrangement and that the NS4A protein intermediate containing the uncleaved C-terminal transmembrane domain plays an essential role in these membrane rearrangements.
Figures
FIG. 1.
Schematic representation of KUNV genes/gene cassettes encoded in the Semliki Forest virus replicon vectors. All constructs were generated via PCR and appropriate restriction digest, and where required, signal sequences were included to ensure correct translocation of the expressed polyproteins into the lumen of the rough endoplasmic reticulum. The NS4A(−2K) C terminus was determined by previous studies (17), and the NS2B-3pro construct contained the first 187 amino acids of NS3 required for efficient in trans proteolytic processing at cytoplasmic dibasic sites within the KUN polyproteins. The 26S promoter represents the subgenomic promoter of pSFV used for expression of foreign genes.
FIG. 2.
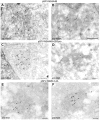
Distinct membrane rearrangements induced upon cytoplasmic replication of either the Semliki Forest virus replicon (A) or the Kunjin virus replicon (B). Abbreviations: CPVI, cytopathic vacuoles characteristic of alphavirus replication; CM/PC, convoluted membranes/paracrystalline arrays characteristic for flavivirus replication. Bars, 500 nm.
FIG. 3.
Expression of KUNV genes/gene cassettes by the SFV replicon vector results in correct processing of the individual proteins and reveals a requirement of the viral protease for cleavage of NS4A and NS4B. BHK cells were electroporated and radiolabeled at 11 h posttransfection with [35S]methionine-cysteine. Individual KUNV proteins were then isolated from the collected lysates by RIP using monospecific rabbit antisera (A and B). Protein migration was assessed against a [35S]methionine-cysteine-radiolabeled lysate generated from KUN-infected BHK cells and by the predicted molecular mass. Note that the expression of the NS2B-3pro construct yields an NS3 species of ∼20 kDa corresponding to the first 187 amino acids of the NS3 protein that is isolated after RIP with anti-NS3 antibodies. Uncleaved NS4A-NS4B polyproteins are indicated by asterisks in panel A. Mock lysates are shown in panel C.
FIG. 4.
Subcellular localization of the expressed KUN proteins as observed by IF. BHK cells transfected as indicated were fixed with 4% paraformaldehyde-0.05% Triton X-100, and distribution of the individual expressed proteins was observed using monospecific rabbit antisera and anti-rabbit FITC-conjugated IgG. Some smaller foci were also observed in cells expressing NS4A (a) and NS4A(−2K) (b). Large perinuclear foci were only observed in cells transfected with NS4A plus NS2B-3pro (c and k), NS4A-4B (d and h), NS4A-4B plus NS2B-3pro (e, i, and l), and NS2B-3-4A (f and m). Also note nuclear translocation of NS4B when expressed alone (g) or when cleaved from the polyproteins (i) but not when the viral protease was absent (h). SFV-vector-only-transfected BHK cells immunostained with the indicated anti-KUNV antibodies served as controls.
FIG. 5.
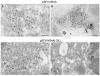
Formation of characteristic flavivirus CM/PC membrane structures upon expression of NS4A4B plus 2B3pro or NS2B-3-4A. Cryosections from transfected BHK cells were immunolabeled with antibodies to NS4A (A to C) or NS3 (D to F). Membrane structures visually analogous to those observed during flavivirus infection were induced during expression of NS4A-4B plus NS2B-3pro (C and D) or NS2B-3-4A (E and F). Similar membrane rearrangements were also observed during expression of NS4A-4B (A and B); however, these latter structures resembled loose chains of vesicles that may represent precursor structures to those observed in the other panels. Arrowheads indicate gold particles binding to the anti-NS3 or anti-NS4A antibodies, and the arrows indicate the individual vesicles within the induced membrane chains. Bars, 200 nm.
FIG. 6.
NS4A proteins with or without the C-terminal 2K peptide can induce the formation of characteristic flavivirus CM/PC membrane structures in Vero cells. Cryosections from Vero cells electroporated with construct pSFV-NS4A or pSFV-NS4A(−2K) were immunolabeled with antibodies to NS4A (A to D) and visualized with 10 nm protein A gold. Membrane structures visually analogous to those observed during flavivirus infection were induced; however, these structures appeared subtly different with observed striations (arrows in panels A and C). Nu, nucleus. Bars, 200 nm.
FIG. 7.
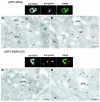
Localization of the mature NS4A protein to the Golgi apparatus early after transfection of Vero cells with the NS4A(−2K) construct. Electroporated cells were fixed at 6.5 h for cryosectioning or at 23 h postelectroporation for IF analysis. Localization of the NS4A proteins (visualized with Oregon green) was compared by immunolabeling to that of the recognized Golgi marker Giantin (visualized with Texas Red). Cryosections revealed an accumulation of gold-labeled anti-NS4A antibodies within visually recognized Golgi bodies only after transfection with the NS4A(−2K) construct (C and D) but not for the NS4A construct (A and B). Arrows indicate the gold-labeled anti-NS4A antibodies Abbreviations: Golgi, Golgi apparatus; Nu, nucleus; rER, rough endoplasmic reticulum. Bars, 200 nm.
Similar articles
- The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner.
Miller S, Kastner S, Krijnse-Locker J, Bühler S, Bartenschlager R. Miller S, et al. J Biol Chem. 2007 Mar 23;282(12):8873-82. doi: 10.1074/jbc.M609919200. Epub 2007 Feb 2. J Biol Chem. 2007. PMID: 17276984 - Determinants in Nonstructural Protein 4A of Dengue Virus Required for RNA Replication and Replication Organelle Biogenesis.
Cortese M, Mulder K, Chatel-Chaix L, Scaturro P, Cerikan B, Plaszczyca A, Haselmann U, Bartenschlager M, Neufeldt CJ, Bartenschlager R. Cortese M, et al. J Virol. 2021 Oct 13;95(21):e0131021. doi: 10.1128/JVI.01310-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379504 Free PMC article. - Cleavage at a novel site in the NS4A region by the yellow fever virus NS2B-3 proteinase is a prerequisite for processing at the downstream 4A/4B signalase site.
Lin C, Amberg SM, Chambers TJ, Rice CM. Lin C, et al. J Virol. 1993 Apr;67(4):2327-35. doi: 10.1128/JVI.67.4.2327-2335.1993. J Virol. 1993. PMID: 8445732 Free PMC article. - Kunjin RNA replication and applications of Kunjin replicons.
Westaway EG, Mackenzie JM, Khromykh AA. Westaway EG, et al. Adv Virus Res. 2003;59:99-140. doi: 10.1016/s0065-3527(03)59004-2. Adv Virus Res. 2003. PMID: 14696328 Review. - [Kunjin virus replicon--a novel viral vector].
Li S, Li X, Qin E, Qin C. Li S, et al. Sheng Wu Gong Cheng Xue Bao. 2011 Feb;27(2):141-6. Sheng Wu Gong Cheng Xue Bao. 2011. PMID: 21650037 Review. Chinese.
Cited by
- A Combined Genetic-Proteomic Approach Identifies Residues within Dengue Virus NS4B Critical for Interaction with NS3 and Viral Replication.
Chatel-Chaix L, Fischl W, Scaturro P, Cortese M, Kallis S, Bartenschlager M, Fischer B, Bartenschlager R. Chatel-Chaix L, et al. J Virol. 2015 Jul;89(14):7170-86. doi: 10.1128/JVI.00867-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926641 Free PMC article. - A three-dimensional comparison of tick-borne flavivirus infection in mammalian and tick cell lines.
Offerdahl DK, Dorward DW, Hansen BT, Bloom ME. Offerdahl DK, et al. PLoS One. 2012;7(10):e47912. doi: 10.1371/journal.pone.0047912. Epub 2012 Oct 24. PLoS One. 2012. PMID: 23112871 Free PMC article. - Proteomic profiling of the TRAF3 interactome network reveals a new role for the ER-to-Golgi transport compartments in innate immunity.
van Zuylen WJ, Doyon P, Clément JF, Khan KA, D'Ambrosio LM, Dô F, St-Amant-Verret M, Wissanji T, Emery G, Gingras AC, Meloche S, Servant MJ. van Zuylen WJ, et al. PLoS Pathog. 2012;8(7):e1002747. doi: 10.1371/journal.ppat.1002747. Epub 2012 Jul 5. PLoS Pathog. 2012. PMID: 22792062 Free PMC article. - Yellow fever: a reemerging threat.
Gardner CL, Ryman KD. Gardner CL, et al. Clin Lab Med. 2010 Mar;30(1):237-60. doi: 10.1016/j.cll.2010.01.001. Clin Lab Med. 2010. PMID: 20513550 Free PMC article. Review. - Bovine viral diarrhea virus NS4B protein is an integral membrane protein associated with Golgi markers and rearranged host membranes.
Weiskircher E, Aligo J, Ning G, Konan KV. Weiskircher E, et al. Virol J. 2009 Nov 3;6:185. doi: 10.1186/1743-422X-6-185. Virol J. 2009. PMID: 19887001 Free PMC article.
References
- Bienz, K., D. Egger, and L. Pasamontes. 1987. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology 160:220-226. - PubMed
- Chu, P. W., and E. G. Westaway. 1992. Molecular and ultrastructural analysis of heavy membrane fractions associated with the replication of Kunjin virus RNA. Arch. Virol. 125:177-191. - PubMed
- Chu, P. W., E. G. Westaway, and G. Coia. 1992. Comparison of centrifugation methods for molecular and morphological analysis of membranes associated with RNA replication of the flavivirus Kunjin. J. Virol. Methods 37:219-234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources