Dendritic cell-based multi-epitope immunotherapy of hormone-refractory prostate carcinoma - PubMed (original) (raw)
Clinical Trial
Dendritic cell-based multi-epitope immunotherapy of hormone-refractory prostate carcinoma
Ying Waeckerle-Men et al. Cancer Immunol Immunother. 2006 Dec.
Abstract
Background: Dendritic cell (DC)-based immunotherapy is a promising approach to augment tumor antigen-specific T cell responses in cancer patients. However, tumor escape with down-regulation or complete loss of target antigens may limit the susceptibility of tumor cells to the immune attack. Concomitant generation of T cell responses against several immunodominant antigens may circumvent this potential drawback. In this trial, we determined the immunostimulatory capacity of autologous DC pulsed with multiple T cell epitopes derived from four different prostate-specific antigens in patients with advanced hormone-refractory prostate cancer.
Patients and methods: Autologous DC of HLA-A*0201(+) patients with hormone-refractory prostate cancer were loaded with antigenic peptides derived from prostate stem cell antigen (PSCA(14-22)), prostatic acid phosphatase (PAP(299-307)), prostate-specific membrane antigen (PSMA(4-12)), and prostate-specific antigen (PSA(154-163)). DC were intradermally applied six times at biweekly intervals followed-in the case of an enhanced immune response-by monthly booster injections. Immune monitoring during the time of ongoing vaccinations (12-59 weeks) included ex vivo ELISPOT measurements, MHC tetramer analysis and in vitro cytotoxicity assays.
Results: Of the initial six patients, three qualified for long-term multi-epitope DC vaccination. This regime was tolerated well by all three patients. The vaccination elicited significant cytotoxic T cell responses against all prostate-specific antigens tested. In addition, memory T cell responses against the control peptides derived from influenza matrix protein and tetanus toxoid were efficiently boosted. Clinically, the long-term DC vaccination was associated with an increase in PSA doubling time.
Conclusions: DC-based multi-epitope immunotherapy with repeated boosting in men with hormone-refractory prostate carcinoma is feasible and generates efficient cellular antitumor responses.
Figures
Fig. 1
Phenotype of DC generated from PBMC of patients with hormone-refractory prostate carcinoma. a Representative surface molecule expression pattern of mature DC from P12. DC were collected after 48 h activation with proinflammatory cytokines and stained with the indicated mAbs for phenotypic analysis with flow cytometry (filled histograms), respectively. Open line histograms represent stainings with isotype matched control antibodies. b In vitro migration capacity of matured DC. Matured DC were tested for CCR7-dependent migration towards CCL19 (dark gray bars) and CCL21 (light gray bars). Medium without cytokines was used to record spontaneous migration (open bars). The percentage of migrated DC was calculated as the mean±SEM of duplicate measurements
Fig. 2
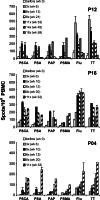
T cell responses of three patients (P04, P12, and P16) to prostate and control antigens as determined by ELISPOT analysis. ELISPOT assays were performed with PBMC obtained at the time points indicated within the panels. Shown are the number of spots per 106 PBMC obtained in the presence of indicated peptides PSCA14–22 (PSCA), PAP299–307 (PAP), PSMA4–12 (PSMA), PSA154–163 (PSA), influenza matrix58–66 (Flu) or tetanus toxoid (TT) after subtraction of the number of spots obtained with the same PBMC in the absence of antigen. The values represent the mean of duplicates±SEM
Fig. 3
In vitro restimulation of antigen-specific CTL obtained from patients after the eighth vaccination and assessment of functional activity. a MHC class I peptide-multimer staining after in vitro restimulation of PBMC of the indicated vaccinated patients. PBMC were restimulated for 7 days with FluM or PSCA peptides, respectively. Values in the upper right quadrant of the dot plot indicate the percentage of multimer-binding CD8+ lymphocytes. The data represent one of three independent experiments with similar results. b Cytotoxic activity of FluM and PSCA-specific CTL, which were stimulated as described in a, was determined in 51Cr release assays using T2 cells as peptide-labeled (closed symbols) or unpulsed (open symbols) target cells. c Cytotoxicity of PSCA-specific CTL to human prostate carcinoma cells. PSCA-specific CTL obtained in two vaccinated patients after one cycle of in vitro restimulation as described in a were tested for their ability to lyse human prostate carcinoma cell lines LNCaP (HLA-A*0201 + and PSCA +) or PC3 (HLA-A*0201 − and PSCA +). LNCaP and PC3 cells were either pulsed with exogenous PSCA or control peptide Flu (for LNCaP only), or were left without peptide pulse. Experiments were performed at an effector/target cell ratio of 30:1. Pooled data from two independent experiments are represented as mean±SEM
Fig. 4
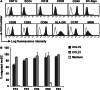
Clinical responses in DC vaccinated patients determined by serial PSA measurements. Linear regression of log-transformed PSA values was used to determine the slopes. PSADT was calculated as indicated in Patients, materials and methods. Arrows indicate time point of vaccination
Similar articles
- Vaccination of advanced prostate cancer patients with PSCA and PSA peptide-loaded dendritic cells induces DTH responses that correlate with superior overall survival.
Thomas-Kaskel AK, Zeiser R, Jochim R, Robbel C, Schultze-Seemann W, Waller CF, Veelken H. Thomas-Kaskel AK, et al. Int J Cancer. 2006 Nov 15;119(10):2428-34. doi: 10.1002/ijc.22097. Int J Cancer. 2006. PMID: 16977630 Clinical Trial. - [Auto-dendritic cell vaccines pulsed with PSA, PSMA and PAP peptides for hormone-refractory prostate cancer].
Zhuang ZX, Shen LQ, Shi Y, Lu X, Shi HZ. Zhuang ZX, et al. Zhonghua Nan Ke Xue. 2010 Aug;16(8):698-704. Zhonghua Nan Ke Xue. 2010. PMID: 21090344 Clinical Trial. Chinese. - Vaccination of hormone-refractory prostate cancer patients with peptide cocktail-loaded dendritic cells: results of a phase I clinical trial.
Fuessel S, Meye A, Schmitz M, Zastrow S, Linné C, Richter K, Löbel B, Hakenberg OW, Hoelig K, Rieber EP, Wirth MP. Fuessel S, et al. Prostate. 2006 Jun 1;66(8):811-21. doi: 10.1002/pros.20404. Prostate. 2006. PMID: 16482569 Clinical Trial. - Immunotherapy for prostate cancer.
Fong L, Small EJ. Fong L, et al. Semin Oncol. 2003 Oct;30(5):649-58. doi: 10.1016/s0093-7754(03)00350-6. Semin Oncol. 2003. PMID: 14571412 Review. - Prostate cancer vaccines: current status and future potential.
Doehn C, Böhmer T, Kausch I, Sommerauer M, Jocham D. Doehn C, et al. BioDrugs. 2008;22(2):71-84. doi: 10.2165/00063030-200822020-00001. BioDrugs. 2008. PMID: 18345705 Review.
Cited by
- Immunotherapy for prostate cancer: lessons from responses to tumor-associated antigens.
Westdorp H, Sköld AE, Snijer BA, Franik S, Mulder SF, Major PP, Foley R, Gerritsen WR, de Vries IJ. Westdorp H, et al. Front Immunol. 2014 May 6;5:191. doi: 10.3389/fimmu.2014.00191. eCollection 2014. Front Immunol. 2014. PMID: 24834066 Free PMC article. Review. - Harnessing Dendritic Cells for Poly (D,L-lactide-_co_-glycolide) Microspheres (PLGA MS)-Mediated Anti-tumor Therapy.
Koerner J, Horvath D, Groettrup M. Koerner J, et al. Front Immunol. 2019 Apr 5;10:707. doi: 10.3389/fimmu.2019.00707. eCollection 2019. Front Immunol. 2019. PMID: 31024545 Free PMC article. Review. - Gene carriers and transfection systems used in the recombination of dendritic cells for effective cancer immunotherapy.
Chen YZ, Yao XL, Tabata Y, Nakagawa S, Gao JQ. Chen YZ, et al. Clin Dev Immunol. 2010;2010:565643. doi: 10.1155/2010/565643. Epub 2010 Dec 20. Clin Dev Immunol. 2010. PMID: 21197274 Free PMC article. Review. - Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration.
Boullart AC, Aarntzen EH, Verdijk P, Jacobs JF, Schuurhuis DH, Benitez-Ribas D, Schreibelt G, van de Rakt MW, Scharenborg NM, de Boer A, Kramer M, Figdor CG, Punt CJ, Adema GJ, de Vries IJ. Boullart AC, et al. Cancer Immunol Immunother. 2008 Nov;57(11):1589-97. doi: 10.1007/s00262-008-0489-2. Epub 2008 Mar 6. Cancer Immunol Immunother. 2008. PMID: 18322684 Free PMC article. - The therapeutic and diagnostic potential of the prostate specific membrane antigen/glutamate carboxypeptidase II (PSMA/GCPII) in cancer and neurological disease.
Evans JC, Malhotra M, Cryan JF, O'Driscoll CM. Evans JC, et al. Br J Pharmacol. 2016 Nov;173(21):3041-3079. doi: 10.1111/bph.13576. Epub 2016 Sep 23. Br J Pharmacol. 2016. PMID: 27526115 Free PMC article. Review.
References
- Jones GW, Mettlin C, Murphy GP, Guinan P, Herr HW, Hussey DH, Chmiel JS, Fremgen AM, Clive RE, Zuber-Ocwieja KE. Patterns of care for carcinoma of the prostate gland: results of a national survey of 1984 and 1990. J Am Coll Surg. 1995;180:545–554. - PubMed
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous