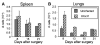Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration - PubMed (original) (raw)
Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration
David J Zammit et al. Immunity. 2006 Apr.
Abstract
Activated virus-specific CD8 T cells remain in the lung airways for several months after influenza virus infection. We show that maintenance of this cell population is dependent upon the route of infection and prolonged presentation of viral antigen in the draining lymph nodes (DLN) of the respiratory tract. The local effects on T cell migration have been examined. We show retention of virus-specific CD8 T cells in the mediastinal lymph node (MLN) and continuing recruitment of blood-borne migrants into the lung airways during antigen presentation. These data show that antigen that is retained after pulmonary influenza virus infection controls the migratory pattern and activation state of virus-specific CD8 T cells near the site of virus amplification.
Figures
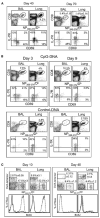
Figure 1. Route of Infection Controls T Cell Activation in the Lung Airways
(A) C57BL/6 mice were given 300 EID50 HKx31 influenza virus by i.n. infection. On days 40 and 70 after infection, NP366–374/Db-specific CD8 T cells in the BAL and parenchyma (Lung) were counted and analyzed for CD69 and IL-7R expression. Percentages of NP366–374/Db-specific cells within the total CD8 T cell populations are shown. (B) Mice were given 5000 EID50 HKx31 influenza virus by i.p. injection. One month later, the mice were treated with 15 μg CpG or control ODN-DNA in the lungs. Gated populations of NP366–374/Db-specific CD8 T cells were analyzed for CD69 and IL-7R expression on days 3 (left) and 9 (right) after ODN-DNA treatment. (C) Mice were primed with LM-OVA i.v. and 1 month later infected with HKx31. Groups of five mice were fed with BrdU water between days 0 and 10 or 30 and 40 after infection. Lymphocytes from the BAL and lung parenchyma were analyzed for NP366–374/Db- and OVA-specific CD8 T cells at the end of the BrdU treatment. Overlaid histograms show BrdU content in gated populations of NP366–374/Db- (dashed lines) and OVA-specific (continuous lines) CD8 T cells. Error bars represent the SEM from five mice.
Figure 2. Different Rates of T Cell Equilibration in Lymphoid and Nonlymphoid Tissues
C57BL/6 mice were given 300 EID50 HKx31 i.n. and 1 month later were joined to uninfected CD45.1+ partner animals. The total number of NP366–374/Db-specific CD8 T cells in the (A) spleens and (B) lungs are shown with standard errors from five pairs of mice.
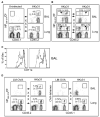
Figure 3. Influenza Virus-Specific, but Not Bystander, CD8 Memory T Cells Continuously Migrate to the Lung Airways
(A and B) C57BL/6 mice were infected with HKx31 and 1 month later were joined to congenic CD45.1+ partner animals that were either (A) uninfected or (B) primed with HKx31 i.n. On day 15 after surgery, five pairs of mice were analyzed for self- and partner-derived NP366–374/Db-specific CD8 T cells in the BAL and lung parenchyma. (A) and (B) show CD8 gate showing percentages of NP366–374/Db-specific cells from the partner animals. The arrows denote the direction of T cell migration. (C) Joined mice that were both infected with HKx31 were analyzed for CD11a expression on NP366–374/Db-specific cells in the lung airways. The CD45.1 marker was used to distinguish partner (continuous line) and self-derived (dashed line) NP366–374/Db-specific CD8 T cells in each animal. In (A), (B), and (C), error bars represent the SEM from five pairs of mice. (D) LM-OVA-infected mice were joined to HKx31-infected partners. Each animal was analyzed for OVA- (right) and NP366–374/Db-specific (left) CD8 T cells in the BAL and lung parenchyma.
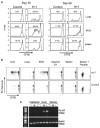
Figure 4. Residual Viral Antigens Persist In Vivo for at Least 2 Months after Influenza Virus Infection
(A) E61-13-H17-infected and control mice received 2 × 3 106 CFSE-labeled CD45.1+ CD8 T cells from the F5-RAG−/− mice by i.v. injection on day 30 (left) or 60 (right) after infection. Eight days after transfer, the NP366–374/Db-specific CD8 T cells in the lungs, MLN, and spleens were analyzed for CD45.1 expression and CFSE intensity. Gated populations of CD45.1+ NP366–374/Db-specific CD8 T cells are shown. Marked regions indicate the percentages of F5 cells that underwent two or more cell divisions after transfer. (B) C57BL/6 mice were infected with E61-13-H17 and 1 month later received 2 × 3 106 CFSE-labeled CD45.1+ RAG−/− F5 cells by i.v. injection. Lymphocytes were isolated from the recipient lungs, inguinal LN, CLN, MLN, and spleens 20 hr after transfer. Lymphocytes were cultured for 3 days in IL-2-supplemented media (20 U/ml). Gated population of CD45.1+ CD8 T cells are shown. Peptide NP366–374 was used as a positive control for cell proliferation. (C) RNA was extracted from the MLN (M), lungs (L), and inguinal lymph nodes (I) from uninfected and E61-13-H17-infected mice 6 (acute) or 33 (memory) days previously. NP and β-actin fragments were amplified from cDNA by PCR. NP expression was detected in the lungs and MLN of acutely infected mice, but not the uninfected controls or memory mice. Multiple experiments gave similar results.
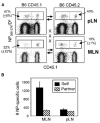
Figure 5. Virus-Specific CD8 T Cells Are Retained in the MLN after Influenza Virus Infection
CD45.2+ C57BL/6 and congenic CD45.1+ partner animals were infected with HKx31 influenza virus and joined 1 month later. On day 15 after surgery, five pairs of mice were analyzed for partner and self-derived NP366–374/Db-specific CD8 T cells in the MLN and other pLN with the CD45.1 and CD45.2 markers. (A) Gated populations of CD8 T cells with percentages of NP366–374/Db -specific cells from the partner animals are shown. (B) The numbers of self- and partner-derived NP366–374/Db-specific CD8 T cells in the MLN and other pLN with SE from five pairs of mice. Error bars represent the SEM from five pairs of mice.
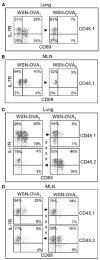
Figure 6. Pulmonary Influenza Virus Infection Is Insufficient for Sustained CD8 T Cell Activation in the Lungs and MLN
WSN-OVA1-infected mice were joined to congenic CD45.1+ partners that had been infected with (A and B) WSN-OVA0 or (C and D) WSN-OVA1. Three weeks after surgery, the lungs (A and C) and MLN (B and D) were analyzed for OVA-specific CD8 T cells that expressed CD69 and IL-7R. The CD45.1 marker was used to distinguish T cells from each animal. Gated populations of CD45.1+ or CD45.2+ OVA-specific CD8 T cells are shown as indicated. Suture marks and arrows denote the direction of T cell migration.
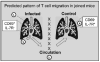
Figure 7. Proposed Pathway of CD8 T Cell Migration in Joined Mice
Virus-specific CD8 T cells that respond to residual viral antigens in the MLN (1) of the influenza virus-infected mice preferentially migrate to the lungs of the donor animals (2) via the pulmonary circulation. Activated (CD69+ IL-R−) CD8 T cells are preferentially retained in the lung tissues of the infected mice, whereas less activated cells pass through and rejoin the circulation (3) where they can gain access to the lungs of the recipient animals (4).
Similar articles
- Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells.
Ho AW, Prabhu N, Betts RJ, Ge MQ, Dai X, Hutchinson PE, Lew FC, Wong KL, Hanson BJ, Macary PA, Kemeny DM. Ho AW, et al. J Immunol. 2011 Dec 1;187(11):6011-21. doi: 10.4049/jimmunol.1100987. Epub 2011 Oct 31. J Immunol. 2011. PMID: 22043017 - The route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen.
Takamura S, Roberts AD, Jelley-Gibbs DM, Wittmer ST, Kohlmeier JE, Woodland DL. Takamura S, et al. J Exp Med. 2010 Jun 7;207(6):1153-60. doi: 10.1084/jem.20090283. Epub 2010 May 10. J Exp Med. 2010. PMID: 20457758 Free PMC article. - Sequential activation of CD8+ T cells in the draining lymph nodes in response to pulmonary virus infection.
Yoon H, Legge KL, Sung SS, Braciale TJ. Yoon H, et al. J Immunol. 2007 Jul 1;179(1):391-9. doi: 10.4049/jimmunol.179.1.391. J Immunol. 2007. PMID: 17579060 - Cutting edge: antigen presentation to CD8 T cells after influenza A virus infection.
Ingulli E, Funatake C, Jacovetty EL, Zanetti M. Ingulli E, et al. J Immunol. 2009 Jan 1;182(1):29-33. doi: 10.4049/jimmunol.182.1.29. J Immunol. 2009. PMID: 19109130 - Viral subversion of B cell responses within secondary lymphoid organs.
Kuka M, Iannacone M. Kuka M, et al. Nat Rev Immunol. 2018 Apr;18(4):255-265. doi: 10.1038/nri.2017.133. Epub 2017 Dec 18. Nat Rev Immunol. 2018. PMID: 29249807 Free PMC article. Review.
Cited by
- Lung influenza virus-specific memory CD4 T cell location and optimal cytokine production are dependent on interactions with lung antigen-presenting cells.
Hargrave KE, Worrell JC, Pirillo C, Brennan E, Masdefiol Garriga A, Gray JI, Purnell T, Roberts EW, MacLeod MKL. Hargrave KE, et al. Mucosal Immunol. 2024 Oct;17(5):843-857. doi: 10.1016/j.mucimm.2024.06.001. Epub 2024 Jun 6. Mucosal Immunol. 2024. PMID: 38851589 Free PMC article. - A specific and portable gene expression program underlies antigen archiving by lymphatic endothelial cells.
Sheridan RM, Doan TA, Lucas C, Forward TS, Uecker-Martin A, Morrison TE, Hesselberth JR, Tamburini BAJ. Sheridan RM, et al. bioRxiv [Preprint]. 2024 Apr 2:2024.04.01.587647. doi: 10.1101/2024.04.01.587647. bioRxiv. 2024. PMID: 38617225 Free PMC article. Preprint. - Immunization-induced antigen archiving enhances local memory CD8+ T cell responses following an unrelated viral infection.
Doan TA, Forward TS, Schafer JB, Lucas ED, Fleming I, Uecker-Martin A, Ayala E, Guthmiller JJ, Hesselberth JR, Morrison TE, Tamburini BAJ. Doan TA, et al. NPJ Vaccines. 2024 Mar 21;9(1):66. doi: 10.1038/s41541-024-00856-6. NPJ Vaccines. 2024. PMID: 38514656 Free PMC article. - Vaccination with mycobacterial lipid loaded nanoparticle leads to lipid antigen persistence and memory differentiation of antigen-specific T cells.
Morgun E, Zhu J, Almunif S, Bobbala S, Aguilar MS, Wang J, Conner K, Cui Y, Cao L, Seshadri C, Scott EA, Wang CR. Morgun E, et al. Elife. 2023 Oct 25;12:RP87431. doi: 10.7554/eLife.87431. Elife. 2023. PMID: 37877801 Free PMC article.
References
- Aronsson F, Karlsson H, Ljunggren HG, Kristensson K. Persistence of the influenza A/WSN/33 virus RNA at midbrain levels of immunodefective mice. J Neurovirol. 2001;7:117–124. - PubMed
- Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlsson H. Persistence of viral RNA in the brain of off-spring to mice infected with influenza A/WSN/33 virus during pregnancy. J Neurovirol. 2002;8:353–357. - PubMed
- Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. - PubMed
- Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–343. - PubMed
Publication types
MeSH terms
Grants and funding
- F32 AI053970/AI/NIAID NIH HHS/United States
- R01 DK045260-10/DK/NIDDK NIH HHS/United States
- AI053970/AI/NIAID NIH HHS/United States
- AI0515 83/AI/NIAID NIH HHS/United States
- AI41576/AI/NIAID NIH HHS/United States
- R01 AI051583/AI/NIAID NIH HHS/United States
- R01 AI051583-04/AI/NIAID NIH HHS/United States
- R01 AI041576-06/AI/NIAID NIH HHS/United States
- R01 DK045260/DK/NIDDK NIH HHS/United States
- R01 AI041576/AI/NIAID NIH HHS/United States
- DK45260/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials