Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors - PubMed (original) (raw)
Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors
Marta Marchetti et al. Mol Biol Cell. 2006 Jul.
Abstract
Type I (alpha/beta) and type II (gamma) interferons (IFNs) bind to distinct receptors, although they activate the same signal transducer and activator of transcription, Stat1, raising the question of how signal specificity is maintained. Here, we have characterized the sorting of IFN receptors (IFN-Rs) at the plasma membrane and the role it plays in IFN-dependent signaling and biological activities. We show that both IFN-alpha and IFN-gamma receptors are internalized by a classical clathrin- and dynamin-dependent endocytic pathway. Although inhibition of clathrin-dependent endocytosis blocked the uptake of IFN-alpha and IFN-gamma receptors, this inhibition only affected IFN-alpha-induced Stat1 and Stat2 signaling. Furthermore, the antiviral and antiproliferative activities induced by IFN-alpha but not IFN-gamma were also affected. Finally, we show that, unlike IFN-alpha receptors, activated IFN-gamma receptors rapidly become enriched in plasma membrane lipid microdomains. We conclude that IFN-R compartmentalization at the plasma membrane, through clathrin-dependent endocytosis and lipid-based microdomains, plays a critical role in the signaling and biological responses induced by IFNs and contributes to establishing specificity within the Jak/Stat signaling pathway.
Figures
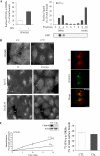
Figure 1.
Plasma membrane compartmentalization and endocytosis of IFNGR1 complexes. (A) IFN-γ stimulates IFNGR complexes association with plasma membrane DRMs. Left, HeLaM cells were incubated at 4°C with 125I-GIR94, a nonneutralizing mAb against IFNGR1, before treatment with (■) or without (□) IFN-γ for 3 min at 37°C. DRMs were prepared, and the percentage of surface IFNGR1 subunits present in DRMs was measured by the amount of radioactivity counted in pooled DRM fractions. GM1 was detected by dot-blot with Cholera toxin–HRP. Right, HeLaM cells were incubated with 125I-IFN-γ for 3 min at 37°C, and sucrose gradient fractionation was performed. The amount of 125I-IFN-γ present in each fraction was expressed as the percentage over total cell surface bound iodinated IFN-γ. Results are representative of at least three independent experiments. (B) Top, the cell surface distribution of IFNGR1 subunits was visualized at 4°C by immunostaining of HeLaM cells. Endocytosis of IFNGR1 was detected by visualizing internalized antibody/IFNGR1 subunits complexes after an acid wash. Right, confocal microscopy analysis of the colocalization of internalized Cy5-Tf and IFNGR1-antibody–bound complexes. Bottom, HeLaM cells were transfected with a plasmid encoding the GFP-Eps15 mutant or with a plasmid encoding the GFP-dyn2 (ba) K44A mutant. In transfected cells (arrows), both Tf and IFNGR1 uptake were inhibited. Scale bars 10 μm. (C) Left, IFNGR1 endocytosis was quantitatively measured in CHC RNAi-treated HeLaM cells using avidin inaccessibility. Inset shows the level of clathrin heavy-chain expression detected by immunoblotting of lysates from RNAi-treated (Clai) and nontreated (Ctl) HeLaM cells. Right, HeLaM cells transfected (■) or not (□) with the CHC RNAi plasmid were incubated for 3 min at 37°C with 125I-IFN-γ, and DRMs were prepared. Radioactivity was counted and the amount of 125I-IFN-γ present in DRMs was expressed as the percentage of total cell surface bound 125I-IFN-γ. Results are representative of at least three independent experiments.
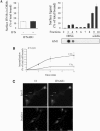
Figure 2.
Plasma membrane compartmentalization and endocytosis of IFNAR1 complexes. (A) Left, IFN-α does not stimulate the association of IFNAR complexes with plasma membrane DRMs. HeLaM cells were incubated at 4°C with 125I-34F10, a nonneutralizing mAb against IFNAR1, before treatment with (■) or without (□) IFN-α for 3 min at 37°C. DRMs were prepared, and the percentage of surface IFNAR1 subunits present in DRMs was measured by the amount of radioactivity counted in pooled DRM fractions. GM1 was detected by dot-blot with Cholera toxin-HRP. Right, HeLaM cells were incubated with 125I-IFN-α for 3 min at 37°C, and sucrose gradient fractionation was performed. The amount of 125I-IFN-α present in each fraction was expressed as a percentage of total cell surface bound iodinated IFN-α. Results are representative of at least three independent experiments. (B) IFNAR1 endocytosis was quantitatively measured in CHC RNAi-treated HeLaM cells using avidin inaccessibility. (C) Eps15 and dynamin are required for endocytosis of IFNAR1. L929R1R2 cells were transfected with a plasmid encoding the GFP-Eps15 mutant or the GFP-dyn2 (ba) K44A mutant. In transfected cells (arrows), both Tf uptake and the endocytosis of IFNAR1 were inhibited. Scale bars, 10 μm.
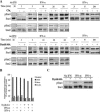
Figure 3.
IFNAR but not IFNGR endocytosis is required for stat activation. (A) Top, HeLaM cells were transfected (+) or not (−) by the CHC RNAi plasmid (Clai) as indicated. Bottom, dynamin HeLa cells were induced (+) or not (−) for the expression of the K44A mutant as indicated. Cells were treated with 1000 U/ml IFN-α2b or IFN-γ at 37°C for the indicated times. Untreated cells are shown for control. Total lysates were analyzed by Western blot/ECL to detect phosphorylated Stat1 (pStat1) and Stat2 (pStat2) as indicated. Results are representative of at least three independent experiments. (B) Dynamin HeLa cells expressing (+) or not (−) K44A dynamin were treated for 3 min at 37°C with or without 1000 U/ml IFN-α2b or IFN-γ as indicated, and plasma membranes were isolated by silica coating. Left, the enrichment of the isolated membranes in different selective enzymatic markers of several cellular compartments including alkaline phosphodiesterase (APDE), β-hexosaminidase (beta-hex), and mannosidase (Manno). Right, equal protein amounts were analyzed in isolated plasma membranes by Western blot/ECL for phospho-Stat1 and Stat1 as indicated. Results are representative of at least three independent experiments.
Figure 4.
IFNAR endocytosis controls the activation of Jak1 and Tyk2. (A) Dynamin HeLa cells expressing (+) or not (−) the K44A mutant were treated for 3 min at 37°C with or without 1000 U/ml IFN-α2b as indicated. Cell lysates were analyzed by Western blot/ECL for Tyk2 and phosphorylated Tyk2 (pTyk2) and for Jak1 and phosphorylated Jak1 (pJak1). Untreated cells are shown for control. (B) Immunoblot images in A were quantified using the NIH Image software, and the ratio of the pTyk2 to Tyk2 and pJak1 to Jak1 signals were plotted to normalize the degree of tyrosine phosphorylation to the total levels of Tyk2 and Jak1 kinases. (C) IFNAR1 and IFNAR2 were immunoprecipitated from dynamin HeLa cells expressing (+) or not (−) the K44A mutant and analyzed by Western blot for their association with Tyk2 and Jak1 kinases, respectively. Blots were stripped and immunoblotted for IFNAR1 and IFNAR2.
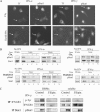
Figure 5.
IFN-R endocytosis is required for IFN-α but not IFN-γ late signaling. (A) Analysis of the nuclear translocation of Stat1. HeLaM cells were treated with the CHC RNAi plasmid (Clai) or transfected with a plasmid encoding the GFP-tagged dynamin K44A mutant as indicated. Cells were treated for 5 min with 1000 U/ml IFN-α2b or IFN-γ and fixed, and the nuclear distribution of phospho-Stat1 was detected by immunofluorescence. Cy3-Tf was cointernalized for 5 min at 37°C to indicate cells inhibited for receptor endocytosis (arrows). Scale bar, 10 μm. Results are representative of at least three independent experiments. (B) Top, HeLaM cells were transfected (+) or not (−) by the CHC RNAi plasmid (Clai) as indicated. Bottom, dynamin HeLa cells were induced (+) or not (−) for the expression of the K44A mutant as indicated. Cells were treated with 1000 U/ml IFN-α2b or IFN-γ at 37°C for 5 and 10 min. After centrifugation, supernatant (C for cytoplasmic) and pellet (N for nuclear) fractions were collected, and equal amounts were immunoblotted for phospho-Stat1 and Stat1, as indicated. Untreated cells are shown for control. (C) Using mAb GIR94, IFNGR1 complexes were immunoprecipitated from the surface of HeLaM cells after treatment or not with IFN-γ and incubated or not with filipin, as indicated. Immunoprecipitates were recovered from DRMs and soluble fractions and resolved on SDS-PAGE analysis as described in Materials and Methods. IFNGR1 immunoprecipitates were immunoblotted for activated IFNGR1 (p-Tyr) and IFNGR1 subunits. Stat1 was immunoprecipitated from soluble and DRM fractions and immunoblotted for tyrosine phosphorylated Stat1 (pStat1).
Figure 6.
Gene transcription and biological responses induced by IFN-α but not IFN-γ are impaired in cells defective for IFN-R endocytosis. (A) HeLaM cells were cotransfected with the ISG54-luciferase or the pGAS-luciferase reporter constructs and the dynamin K44A plasmid. After 48 h, cells were treated with 1000U/ml recombinant IFN-α2b or IFN-γ for 8 h. Luciferase activity was quantified in cell lysates and expressed as the percentage of total activity measured in mock plasmid-transfected cells. The experiments were performed at least three times. (B) The antiviral response was analyzed by using a cytopathic effect reduction assay in cells infected with vsv. Top, HeLaM cells were transfected (□) or not (■) with the CHC RNAi plasmid (Clai). Bottom, dynamin HeLa cells were induced (□) or not (■) for dynamin K44A mutant expression. Cells were treated for 24 h with 1:2 serial dilutions of a 2000 U/ml starting concentration of IFN-α2b or IFN-γ as indicated, and vsv was added for 24 h. Cells were stained with crystal violet, and absorbance at 595 nM was measured. Results are the means of three independent experiments and are represented as a function of serial dilutions of IFN. (C) HeLaM cells transfected (□) or not (■) by the CHC RNAi plasmid (Clai) were starved for 24 h and incubated with 1000 or 100 U/ml IFN-α2b or IFN-γ for 72 h. Results are the means of two independent experiments and are expressed as the percentage of growth inhibition in cells treated with IFN in comparison to nontreated cells.
Similar articles
- Interfering with interferon receptor sorting and trafficking: impact on signaling.
Claudinon J, Monier MN, Lamaze C. Claudinon J, et al. Biochimie. 2007 Jun-Jul;89(6-7):735-43. doi: 10.1016/j.biochi.2007.03.014. Epub 2007 Apr 2. Biochimie. 2007. PMID: 17493737 Review. - Palmitoylation of interferon-alpha (IFN-alpha) receptor subunit IFNAR1 is required for the activation of Stat1 and Stat2 by IFN-alpha.
Claudinon J, Gonnord P, Beslard E, Marchetti M, Mitchell K, Boularan C, Johannes L, Eid P, Lamaze C. Claudinon J, et al. J Biol Chem. 2009 Sep 4;284(36):24328-40. doi: 10.1074/jbc.M109.021915. Epub 2009 Jun 26. J Biol Chem. 2009. PMID: 19561067 Free PMC article. - Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway.
You M, Yu DH, Feng GS. You M, et al. Mol Cell Biol. 1999 Mar;19(3):2416-24. doi: 10.1128/MCB.19.3.2416. Mol Cell Biol. 1999. PMID: 10022928 Free PMC article. - A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.
Michalska A, Blaszczyk K, Wesoly J, Bluyssen HAR. Michalska A, et al. Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
Cited by
- Bacterial and fungal pattern recognition receptors in homologous innate signaling pathways of insects and mammals.
Stokes BA, Yadav S, Shokal U, Smith LC, Eleftherianos I. Stokes BA, et al. Front Microbiol. 2015 Jan 28;6:19. doi: 10.3389/fmicb.2015.00019. eCollection 2015. Front Microbiol. 2015. PMID: 25674081 Free PMC article. Review. - Molecular and cellular factors determining the functional pleiotropy of cytokines.
McFarlane A, Pohler E, Moraga I. McFarlane A, et al. FEBS J. 2023 May;290(10):2525-2552. doi: 10.1111/febs.16420. Epub 2022 Mar 14. FEBS J. 2023. PMID: 35246947 Free PMC article. Review. - Human T-cell leukemia virus type 1 blunts signaling by interferon alpha.
Feng X, Ratner L. Feng X, et al. Virology. 2008 Apr 25;374(1):210-6. doi: 10.1016/j.virol.2007.12.036. Epub 2008 Jan 29. Virology. 2008. PMID: 18234266 Free PMC article. - Separate endocytic pathways regulate IL-5 receptor internalization and signaling.
Lei JT, Martinez-Moczygemba M. Lei JT, et al. J Leukoc Biol. 2008 Aug;84(2):499-509. doi: 10.1189/jlb.1207828. Epub 2008 May 29. J Leukoc Biol. 2008. PMID: 18511572 Free PMC article. - Viral dedication to vigorous destruction of interferon receptors.
Xia C, Anderson P, Hahm B. Xia C, et al. Virology. 2018 Sep;522:19-26. doi: 10.1016/j.virol.2018.06.017. Epub 2018 Jul 6. Virology. 2018. PMID: 30014854 Free PMC article. Review.
References
- Bach E. A., Aguet M., Schreiber R. D. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 1997;15:563–591. - PubMed
- Brodsky F. M., Chen C. Y., Knuehl C., Towler M. C., Wakeham D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell. Dev. Biol. 2001;17:517–568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous