DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases - PubMed (original) (raw)
DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases
Teresa Morales-Ruiz et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Cytosine methylation is an epigenetic mark that promotes gene silencing and plays important roles in development and genome defense against transposons. Methylation patterns are established and maintained by DNA methyltransferases that catalyze transfer of a methyl group from S-adenosyl-L-methionine to cytosine bases in DNA. Erasure of cytosine methylation occurs during development, but the enzymatic basis of active demethylation remains controversial. In Arabidopsis thaliana, DEMETER (DME) activates the maternal expression of two imprinted genes silenced by methylation, and REPRESSOR OF SILENCING 1 (ROS1) is required for release of transcriptional silencing of a hypermethylated transgene. DME and ROS1 encode two closely related DNA glycosylase domain proteins, but it is unknown whether they participate directly in a DNA demethylation process or counteract silencing through an indirect effect on chromatin structure. Here we show that DME and ROS1 catalyze the release of 5-methylcytosine (5-meC) from DNA by a glycosylase/lyase mechanism. Both enzymes also remove thymine, but not uracil, mismatched to guanine. DME and ROS1 show a preference for 5-meC over thymine in the symmetric dinucleotide CpG context, where most plant DNA methylation occurs. Nevertheless, they also have significant activity on both substrates at CpApG and asymmetric sequences, which are additional methylation targets in plant genomes. These findings suggest that a function of ROS1 and DME is to initiate erasure of 5-meC through a base excision repair process and provide strong biochemical evidence for the existence of an active DNA demethylation pathway in plants.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
DME and ROS1 contain a DNA glycosylase domain and are closely related. (Upper) Diagram of DME and ROS1 showing the conserved domains as colored sections. The percentage of identical residues between both proteins is shown above each region. (Lower) Amino acid sequence alignment of DME (amino acids 1519–1659) and ROS1 (amino acids 928-1068) with A. thaliana Nth1, E. coli Nth, and Homo sapiens MutY and Ogg1. GenBank accession nos. are ABC61677, AAP37178, CAC16135, P20625, Q9UIF7, and O15527, respectively. An asterisk marks the lysine residue that is diagnostic of a glycosylase/lyase activity, the filled triangle indicates the conserved aspartic acid residue in the active site, and the open diamonds label the cysteine residues that in E. coli Nth1 ligate a [4Fe–4S] cluster.
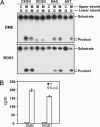
Fig. 2.
DME and ROS1 are 5-meC DNA glycosylases. (A) Double-stranded oligonucleotide substrates containing 5-meC (M) in CpG (CXGG) and non-CpG (XCGG, XAG, and AXT) sequence contexts or the corresponding unmethylated controls were incubated with purified DME (Upper) or ROS1 (Lower) as described in Materials and Methods. Reaction products were separated in a 12% denaturing polyacrylamide gel and visualized by autoradiography. (B) Release of 5-meC from methylated DNA. Plasmid DNA methylated with S-adenosyl-
l
-[methyl-3H]methionine (1,200 cpm) was incubated with purified DME or ROS1, and the released ethanol-soluble material was analyzed by two-dimensional TLC (see Materials and Methods). Distribution of radioactivity between thymine (filled bars) and 5-meC (open bars) was assayed by scintillation counting. The mean of duplicate experiments and their standard errors are shown.
Fig. 3.
DME and ROS1 excise 5-meC and thymine but not uracil. (A) Double-stranded oligonucleotide substrates containing 5-mec (M), T·G, or U·G mismatches in CpG (CXGG) and non-CpG (XCGG, XAG, and AXT) sequence contexts were incubated with purified DME (Upper) or ROS1 (Lower) as described in Materials and Methods. Reaction products were separated in a 12% denaturing polyacrylamide gel and visualized by autoradiography. (B) Double-stranded oligonucleotide substrates containing 5-meC (M), T·G, or U·G mismatches in a CpG sequence context were incubated for 24 h with purified WT DME or mutant DME D1562A (Upper) or WT ROS1 or mutant ROS1 D971A (Lower). Reaction products were separated in a 12% denaturing polyacrylamide gel and visualized by autoradiography.
Fig. 4.
Kinetics of DME and ROS1 action on 5-meC and thymine in CpG and non-CpG sequence contexts. The time-dependent generation of incised oligonucleotides was measured by incubating purified DME or ROS1 with double-stranded oligonucleotide substrates containing a 5-meC·G pair (•) or a T·G mismatch (○) in CpG (CXGG) and non-CpG (XCGG, XAG, and AXT) sequence contexts. Reactions were stopped at the indicated times, products were separated in a 12% denaturing polyacrylamide gel, and the relative amount of incised oligonucleotide was quantitated by phosphor imaging.
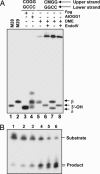
Fig. 5.
DME and ROS1 process 5-meC by a DNA glycosylase/lyase mechanism proceeding through a transient imine intermediate. Double-stranded oligonucleotide substrates containing a C·G, 5-meC·G, or 8-oxoG·C pair in a CpG context were incubated with purified DME (lanes 1, 4, and 7), ROS1 (lanes 2, 5, and 8), or AtOGG1 (lanes 3, 6, and 9) in the presence of NaBH4. After incubation, the reaction mixture was subjected to SDS/PAGE, and products were visualized by autoradiography. Free substrates and crosslinked enzyme–substrate complexes are indicated by arrows. M, 5-meC; O, 8-oxoG.
Fig. 6.
Products formed by DME upon excision of 5-meC. (A) Double-stranded oligonucleotide substrates containing a 5-meC·G (lanes 5–8) or an 8-oxoG·C pair (lanes 3 and 4) in a CpG context were incubated with purified Fpg (lane 3), AtOGG1 (lane 4), or DME (lanes 5–8), and reaction mixtures were separated in a denaturing polyacrylamide sequencing gel (40 × 20 cm). The products formed by DME were further treated with endonuclease IV (lanes 6 and 8) to analyze the nature of 3′ termini. Substrates and enzymes used are indicated at the top of the gel. Oligonucleotide markers of 28 and 29 nucleotides were loaded in lanes 1 and 2, respectively. The β- and δ-elimination products and those carrying 3′-OH termini are indicated by arrows. (B) A double-stranded oligonucleotide substrate containing a 5-meC·G pair in a CpG sequence context was incubated with purified DME, and reactions were stopped at different times. Lanes 1–6 correspond to reaction times of 0.25, 0.5, 1, 2, 10, and 24 h, respectively. Products were separated in a 12% denaturing polyacrylamide gel and visualized by autoradiography.
Similar articles
- Excision of 5-hydroxymethylcytosine by DEMETER family DNA glycosylases.
Jang H, Shin H, Eichman BF, Huh JH. Jang H, et al. Biochem Biophys Res Commun. 2014 Apr 18;446(4):1067-72. doi: 10.1016/j.bbrc.2014.03.060. Epub 2014 Mar 21. Biochem Biophys Res Commun. 2014. PMID: 24661881 Free PMC article. - A discontinuous DNA glycosylase domain in a family of enzymes that excise 5-methylcytosine.
Ponferrada-Marín MI, Parrilla-Doblas JT, Roldán-Arjona T, Ariza RR. Ponferrada-Marín MI, et al. Nucleic Acids Res. 2011 Mar;39(4):1473-84. doi: 10.1093/nar/gkq982. Epub 2010 Oct 29. Nucleic Acids Res. 2011. PMID: 21036872 Free PMC article. - DEMETER plays a role in DNA demethylation and disease response in somatic tissues of Arabidopsis.
Schumann U, Lee JM, Smith NA, Zhong C, Zhu JK, Dennis ES, Millar AA, Wang MB. Schumann U, et al. Epigenetics. 2019 Nov;14(11):1074-1087. doi: 10.1080/15592294.2019.1631113. Epub 2019 Jun 19. Epigenetics. 2019. PMID: 31189415 Free PMC article. - Preventing transcriptional gene silencing by active DNA demethylation.
Kapoor A, Agius F, Zhu JK. Kapoor A, et al. FEBS Lett. 2005 Oct 31;579(26):5889-98. doi: 10.1016/j.febslet.2005.08.039. Epub 2005 Aug 31. FEBS Lett. 2005. PMID: 16162337 Review. - DNA demethylation: a lesson from the garden.
Ikeda Y, Kinoshita T. Ikeda Y, et al. Chromosoma. 2009 Feb;118(1):37-41. doi: 10.1007/s00412-008-0183-3. Epub 2008 Oct 7. Chromosoma. 2009. PMID: 18839198 Review.
Cited by
- Control of DNA demethylation by superoxide anion in plant stem cells.
Wang S, Liu M, Hu D, Dong Z, Zhao Z. Wang S, et al. Nat Chem Biol. 2024 Sep 12. doi: 10.1038/s41589-024-01737-8. Online ahead of print. Nat Chem Biol. 2024. PMID: 39266722 - Epigenetic gene regulation in plants and its potential applications in crop improvement.
Zhang H, Zhu JK. Zhang H, et al. Nat Rev Mol Cell Biol. 2025 Jan;26(1):51-67. doi: 10.1038/s41580-024-00769-1. Epub 2024 Aug 27. Nat Rev Mol Cell Biol. 2025. PMID: 39192154 Review. - aChIP is an efficient and sensitive ChIP-seq technique for economically important plant organs.
Zhang Q, Zhong W, Zhu G, Cheng L, Yin C, Deng L, Yang Y, Zhang Z, Shen J, Fu T, Zhu JK, Zhao L. Zhang Q, et al. Nat Plants. 2024 Sep;10(9):1317-1329. doi: 10.1038/s41477-024-01743-7. Epub 2024 Aug 23. Nat Plants. 2024. PMID: 39179701 - Genome-Wide Analysis of DNA Demethylases in Land Plants and Their Expression Pattern in Rice.
Mao S, Xiao J, Zhao Y, Hou J, Li L. Mao S, et al. Plants (Basel). 2024 Jul 26;13(15):2068. doi: 10.3390/plants13152068. Plants (Basel). 2024. PMID: 39124186 Free PMC article. - Recent Advances in Studies of Genomic DNA Methylation and Its Involvement in Regulating Drought Stress Response in Crops.
Fan Y, Sun C, Yan K, Li P, Hein I, Gilroy EM, Kear P, Bi Z, Yao P, Liu Z, Liu Y, Bai J. Fan Y, et al. Plants (Basel). 2024 May 17;13(10):1400. doi: 10.3390/plants13101400. Plants (Basel). 2024. PMID: 38794470 Free PMC article. Review.
References
- Bender J. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2004;55:41–68. - PubMed
- Bird A. Genes Dev. 2002;16:6–21. - PubMed
- Colot V., Rossignol J. L. BioEssays. 1999;21:402–411. - PubMed
- Holliday R., Pugh J. E. Science. 1975;187:226–232. - PubMed
- Riggs A. D. Cytogenet. Cell Genet. 1975;14:9–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials