Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury - PubMed (original) (raw)
Comparative Study
Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury
James M Massey et al. J Neurosci. 2006.
Abstract
Upregulation of extracellular chondroitin sulfate proteoglycans (CSPGs) after CNS injuries contributes to the impediment of functional recovery by restricting both axonal regeneration and synaptic plasticity. In the present study, the effect of degrading CSPGs with the application of the bacterial enzyme chondroitinase ABC (chABC) into the cuneate nucleus of rats partially denervated of forepaw dorsal column axons was examined. A dorsal column transection between the C6-C7 dorsal root entry zones was followed immediately by an ipsilateral brainstem injection of either chABC or a bacterial-derived control enzyme [penicillinase (P-ase)] and then subsequently (1 week later) followed with a second brainstem enzyme injection and cholera toxin B subunit (CTB) tracer injection into the ipsilateral forepaw digits and pads. After 1 additional week, the rats underwent electrophysiological receptive field mapping of the cuneate nucleus and/or anatomical evaluation. Examination of the brainstems of rats from each group revealed that CSPGs had been reduced after chABC treatment. Importantly, in the chABC-treated rats (but not in the P-ase controls), a significantly greater area of the cuneate nucleus was occupied by physiologically active CTB traced forepaw afferents that had been spared by the initial cord lesion. These results demonstrate, for the first time, a functional change directly linked to anatomical evidence of sprouting by spinal cord afferents after chABC treatment.
Figures
Figure 1.
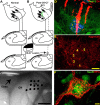
Diagram summary of the experimental design. A, Normal forepaw primary afferents in the rat cuneate nuclei are distributed in a somatotopic arrangement. B, To assess the effect of chABC digestion on collateral sprouting in the cuneate nuclei, the dorsal columns were transected between the C6 and C7 root entry zones denervating all primary afferent input from digits 4 and 5 plus most of the input from digit 3. chABC was then injected adjacent to the ipsilateral cuneate nuclei. Microelectrode receptive field mapping was used in combination with CTB injections into the palmar digits and footpads to demonstrate the functional and anatomical distribution of forepaw primary afferent terminals. C, Image of the exposed brainstem showing the dorsal surface of the dorsal column nuclei from a sham rat that underwent electrophysiological assessment. Electrode entry points into the cuneate nuclei (CN) are marked by the black dots. GN, Gracile nucleus. D, Each electrode was coated with DiI (red), which allowed anatomical localization of the recording penetrations in transverse sections labeled for cuneate nuclei neurons and primary afferent anatomy by MAP2-IR (green) and CTB-IR (blue). E, Aggrecan-IR using the Cat-301 antibody labels perineuronal nets and extracellular matrix in the normal cuneate nuclei. F, Using high-power confocal microscopy, CSPGs contained in the perineuronal net labeled by WFA cytochemistry (red) form a lace-like cloak around NeuN-immunoreactive (green) cuneate nuclei neurons. Numbers 1–5 denote the normal locations of the digit terminal fields. Scale bars: C, 1 mm; D, 200 μm; E, 100 μm; F, 10 μm.
Figure 2.
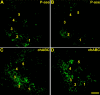
Sections through a normal rat brainstem demonstrating the effectiveness of chABC injections. chABC effectively reduces glycosaminoglycans on CSPGs in the normal cuneate nucleus (CN) and nearby areas of the medulla after in vivo injections into the caudal brainstem. WFA (green) and 2B6 (stub antibody, red) label complementary areas and demonstrate the extent of chABC digestion of glycosaminoglycan moieties (A–E). Both aggrecan (Cat-301; red, F, H, I; compare with Fig. 1_E_) and neurocan (1F6; red, G, J) core protein immunoreactivity was also reduced in areas heavily labeled by 2B6 immunoreactivity in adjacent sections. At higher power, borders of glycosaminoglycan chain digestion (absence of green WFA staining) next to intact CSPGs (presence of WFA green staining) shared similar borders with the reduction of CSPG core proteins (H, I). The dotted white line demonstrates the area of the cuneate nucleus (A, F, G). ECN, External cuneate nucleus; GN, gracile nucleus. Scale bar: A, B, F, G, 200 μm; C–E, H–J, 60 μm.
Figure 3.
After a C6–C7 SCI and two injections of either chABC (A) or P-ase (B; at the time of cord injury and 1 week later), evidence of enzyme digestion demonstrated by 2B6-immunoreactive labeling of C4 sulfated glycosaminoglycan “stubs” similar to that seen in normal rats (see Fig. 2) was observed in chABC-treated (A) but not in P-ase-treated (B) injured rats. CN, Cuneate nucleus; GN, gracile nucleus. Scale bar, 200 μm.
Figure 4.
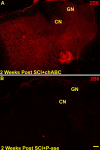
Representative confocal images of CTB traced forepaw primary afferent terminal fields in the cuneate nuclei of two P-ase-treated or two chABC-treated rats after C6–C7 spinal cord injuries (A–D). The CTB-IR remaining in the P-ase-treated rats was primarily confined to the normal somatotopic locations of digits 1 and 2 (A, B). This coincided with the receptive fields observed in rats that underwent electrophysiological assessment (see Figs. 5, 6). In chABC-treated injured rats, the CTB-IR was significantly more extensive than that observed in injured rats treated with P-ase (E), spreading throughout adjacent digit locations and often well into the dorsalmost surface of the cuneate nuclei (C, D). Numbers 1–5 denote the normal locations of the digit terminal fields. Scale bar, 50 μm.
Figure 5.
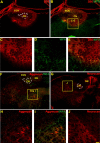
Images of representative sections through the cuneate nuclei and their matching receptive field maps from sham (A, B), P-ase-treated (C, D) or chABC-treated (E, F) rats. MAP2-IR (green) and CTB-IR (blue) demonstrate the anatomical features and traced terminal field locations in relationship to the recording site locations observed along the microelectrode trajectory. Activity elicited by stimulation to the ipsilateral body surfaces revealed an orderly progression of receptive fields (black) dominated by areas around each of the digits and forepaw in the non-injured rats (B). After the C6–C7 spinal cord injury, the receptive fields were limited primarily to the digits 1 and 2 in both the P-ase-treated (D) and chABC-treated (F) injured rats. However, in the chABC-treated injured rats, these receptive fields were far more common (see Fig. 6) and could be mapped well into ectopic locations in the cuneate nucleus often located adjacent to areas of CTB-IR (E, F). Silent areas (*), in which no activity could be elicited by stimulation to any body surface, were seen in both groups of injured rats but were far more common in the P-ase-treated rats (D, F; see Fig. 6_B_). Numbers 1–5 denote the normal locations of the digit terminal fields. Scale bar, 100 μm.
Figure 6.
Quantification of receptive field frequencies from sham, P-ase-treated, and chABC-treated rats. The frequency of cuneate neuron activation in response to forepaw stimulation in the chABC-treated rats was significantly greater than that observed in P-ase-treated rats after C6–C7 SCI (A). Additionally, there was a significant reduction in the number of recording sites in chABC-treated injured rats that could not be activated by any body surface when compared with P-ase-treated injured rats (B). Digits 1 and 2 receptive field frequencies were significantly increased in the chABC-treated injured rats but not in the P-ase-treated injured rats demonstrating that the expansion of territories from the primary afferents originating from these areas of the forepaw were responsible for much of the increases in cuneate activation (C). Data considered for statistical comparisons are shown by brackets: *p < 0.05; ***p < 0.001.
Similar articles
- Administration of chondroitinase ABC rostral or caudal to a spinal cord injury site promotes anatomical but not functional plasticity.
Tom VJ, Kadakia R, Santi L, Houlé JD. Tom VJ, et al. J Neurotrauma. 2009 Dec;26(12):2323-33. doi: 10.1089/neu.2009.1047. J Neurotrauma. 2009. PMID: 19659409 Free PMC article. - Chondroitinase ABC-mediated plasticity of spinal sensory function.
Cafferty WB, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, McMahon SB. Cafferty WB, et al. J Neurosci. 2008 Nov 12;28(46):11998-2009. doi: 10.1523/JNEUROSCI.3877-08.2008. J Neurosci. 2008. PMID: 19005065 Free PMC article. - Benefit of chondroitinase ABC on sensory axon regeneration in a laceration model of spinal cord injury in the rat.
Shields LB, Zhang YP, Burke DA, Gray R, Shields CB. Shields LB, et al. Surg Neurol. 2008 Jun;69(6):568-77; discussion 577. doi: 10.1016/j.surneu.2008.02.009. Surg Neurol. 2008. PMID: 18486695 Free PMC article. - Proteoglycans in the central nervous system: plasticity, regeneration and their stimulation with chondroitinase ABC.
Kwok JC, Afshari F, García-Alías G, Fawcett JW. Kwok JC, et al. Restor Neurol Neurosci. 2008;26(2-3):131-45. Restor Neurol Neurosci. 2008. PMID: 18820407 Review. - Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury.
Bradbury EJ, Carter LM. Bradbury EJ, et al. Brain Res Bull. 2011 Mar 10;84(4-5):306-16. doi: 10.1016/j.brainresbull.2010.06.015. Epub 2010 Jul 8. Brain Res Bull. 2011. PMID: 20620201 Review.
Cited by
- Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice.
Starkey ML, Bartus K, Barritt AW, Bradbury EJ. Starkey ML, et al. Eur J Neurosci. 2012 Dec;36(12):3665-78. doi: 10.1111/ejn.12017. Epub 2012 Oct 14. Eur J Neurosci. 2012. PMID: 23061434 Free PMC article. - Overcoming macrophage-mediated axonal dieback following CNS injury.
Busch SA, Horn KP, Silver DJ, Silver J. Busch SA, et al. J Neurosci. 2009 Aug 12;29(32):9967-76. doi: 10.1523/JNEUROSCI.1151-09.2009. J Neurosci. 2009. PMID: 19675231 Free PMC article. - Central nervous system regeneration.
Varadarajan SG, Hunyara JL, Hamilton NR, Kolodkin AL, Huberman AD. Varadarajan SG, et al. Cell. 2022 Jan 6;185(1):77-94. doi: 10.1016/j.cell.2021.10.029. Cell. 2022. PMID: 34995518 Free PMC article. Review. - Chondroitinase ABC Administration Facilitates Serotonergic Innervation of Motoneurons in Rats With Complete Spinal Cord Transection.
Takiguchi M, Miyashita K, Yamazaki K, Funakoshi K. Takiguchi M, et al. Front Integr Neurosci. 2022 Jun 30;16:881632. doi: 10.3389/fnint.2022.881632. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35845919 Free PMC article. - Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a beneficial inflammatory response following spinal cord injury.
Dyck S, Kataria H, Alizadeh A, Santhosh KT, Lang B, Silver J, Karimi-Abdolrezaee S. Dyck S, et al. J Neuroinflammation. 2018 Mar 20;15(1):90. doi: 10.1186/s12974-018-1128-2. J Neuroinflammation. 2018. PMID: 29558941 Free PMC article.
References
- Allen NJ, Barres BA (2005). Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol 15:542–548. - PubMed
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB (2002). Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640. - PubMed
- Bruckner G, Bringmann A, Hartig W, Koppe G, Delpech B, Brauer K (1998). Acute and long-lasting changes in extracellular-matrix chondroitin-sulphate proteoglycans induced by injection of chondroitinase ABC in the adult rat brain. Exp Brain Res 121:300–310. - PubMed
- Buffo A, Zagrebelsky M, Huber AB, Skerra A, Schwab ME, Strata P, Rossi F (2000). Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons. J Neurosci 20:2275–2286. - PMC - PubMed
- Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA (2005). Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma 22:226–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous