Kinetics and synergistic effects of siRNAs targeting structural and replicase genes of SARS-associated coronavirus - PubMed (original) (raw)
Kinetics and synergistic effects of siRNAs targeting structural and replicase genes of SARS-associated coronavirus
Ming-Liang He et al. FEBS Lett. 2006.
Abstract
SARS-associated coronavirus was identified as the etiological agent of severe acute respiratory syndrome and a large virus pool was identified in wild animals. Virus generates drug resistance through fast mutagenesis and escapes antiviral treatment. siRNAs targeting different genes would be an alternative for overcoming drug resistance. Here, we report effective siRNAs targeting structural genes (i.e., spike, envelope, membrane, and nucleocapsid) and their antiviral kinetics. We also showed the synergistic effects of two siRNAs targeting different functional genes at a very low dose. Our findings may pave a way to develop cost effective siRNA agents for antiviral therapy in the future.
Figures
Figure 1
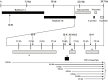
The diagram of the effective siRNA's targeting sites. SCoV genomic RNA is composed of 30‐kb nucleotides. The replicase gene, which comprises about 60% of the genome, encodes two polyproteins that undergo cotranslational proteolytic processing. The downstream sequence of the replicase gene encodes four structural proteins (spike, envelope, membrane, and nucleocapsid) and multiple potential nonstructure proteins (not shown). The SCoV replicase gene products are directly translated from genomic RNA, while the remaining viral proteins are translated from subgenomic transcripts. The target sites of the effective siRNAs are shown by arrows. Si‐ = SARSi‐.
Figure 2
Inhibition of CPE by siRNAs. Cytopathic effects: FRhk‐4 cells were transfected with (III–VIII, 200 nM) or without (I and II) siRNAs and infected with SCoV (II and IV–VIII). The photos were taken under phase‐contrast microscope at 36 h post‐infection. The arrows show cytopathic cells.
Figure 3
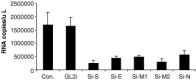
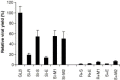
Reduction of intracellular viral genomic RNA copies by siRNA. The cellular RNA was isolated and quantitative RT‐PCR experiments were conducted 24 h post‐infection. The experiments were performed in triplicate and repeated at least three times. Detection revealed that these siRNAs reduced viral replication effectively (student's t test, P< 0.01). The values (mean ± S.E.) were shown in a typical experiment. The values are: GL2i, 1.69 × 106± 4.7 × 103; SARSi‐R, 1.2 × 105± 5.5 × 102; SARSi‐S, 2.5 × 105± 1.0 × 105; SARSi‐E, 4.4 × 105± 1.0 × 105; SARSi‐M1, 3.8 × 105± 2.2 × 105; SARSi‐M2, 3.1 × 105± 1.2 × 105; and SARSi‐N, 5.7 × 105± 1.5 × 105.
Figure 4
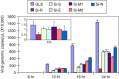
The kinetics of viral genomic RNA. FRhk‐4 cells were transfected with siRNAs and infected with SCoV. At 1 h post‐infection the medium containing viruses was removed. The cells were then washed twice with PBS containing 5 mM EDTA, and cultured in MEM medium containing 1% FBS. Total RNA was isolated, and viral genomic copies were quantified by real‐time RT‐PCR.
Figure 5
Dose‐dependent inhibition of SCoV reproduction by siRNAs. Different amounts of siRNAs targeting SCoV and unrelated siRNA GL2i (for normalization of transfection efficiency) were transfected into FRhk‐4 cells. At 24 h post‐transfection the viral titers in the conditioned media were measured by back‐titration. The value of control (GL2i only) was defined as 100. The values (means ± S.D.) represent the average from the three independent experiments.
Figure 6
The effects of combined siRNAs. A single siRNA (10 nM), two combined siRNAs (5 nM each siRNA) were transfected into FRhk‐4 cells. At 6 h post‐transfection the medium was removed and the cells were infected with SCoV suspended in DMEM for 1 h. Then the medium was replaced with DMEM with 1% of FBS. At 24 h post‐infection the viral titers in the conditioned medium were determined by virus infectivity assay. The viral titer of GL2i samples was defined as 100. The values (means ±S.D.) represent the average from three independent experiments.
Similar articles
- Prophylactic and therapeutic effects of small interfering RNA targeting SARS-coronavirus.
Zheng BJ, Guan Y, Tang Q, Du C, Xie FY, He ML, Chan KW, Wong KL, Lader E, Woodle MC, Lu PY, Li B, Zhong N. Zheng BJ, et al. Antivir Ther. 2004 Jun;9(3):365-74. Antivir Ther. 2004. PMID: 15259899 - siRNA targeting the leader sequence of SARS-CoV inhibits virus replication.
Li T, Zhang Y, Fu L, Yu C, Li X, Li Y, Zhang X, Rong Z, Wang Y, Ning H, Liang R, Chen W, Babiuk LA, Chang Z. Li T, et al. Gene Ther. 2005 May;12(9):751-61. doi: 10.1038/sj.gt.3302479. Gene Ther. 2005. PMID: 15772689 Free PMC article. - Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins.
Prentice E, McAuliffe J, Lu X, Subbarao K, Denison MR. Prentice E, et al. J Virol. 2004 Sep;78(18):9977-86. doi: 10.1128/JVI.78.18.9977-9986.2004. J Virol. 2004. PMID: 15331731 Free PMC article. - Characterization of viral proteins encoded by the SARS-coronavirus genome.
Tan YJ, Lim SG, Hong W. Tan YJ, et al. Antiviral Res. 2005 Feb;65(2):69-78. doi: 10.1016/j.antiviral.2004.10.001. Antiviral Res. 2005. PMID: 15708633 Free PMC article. Review. - Recent developments in the virology and antiviral research of severe acute respiratory syndrome coronavirus.
Yeung KS, Meanwell NA. Yeung KS, et al. Infect Disord Drug Targets. 2007 Mar;7(1):29-41. doi: 10.2174/187152607780090739. Infect Disord Drug Targets. 2007. PMID: 17346209 Review.
Cited by
- Glucose-regulated protein 78 is an intracellular antiviral factor against hepatitis B virus.
Ma Y, Yu J, Chan HL, Chen YC, Wang H, Chen Y, Chan CY, Go MY, Tsai SN, Ngai SM, To KF, Tong JH, He QY, Sung JJ, Kung HF, Cheng CH, He ML. Ma Y, et al. Mol Cell Proteomics. 2009 Nov;8(11):2582-94. doi: 10.1074/mcp.M900180-MCP200. Epub 2009 Aug 11. Mol Cell Proteomics. 2009. PMID: 19671925 Free PMC article. - Transcriptional profiling of Vero E6 cells over-expressing SARS-CoV S2 subunit: insights on viral regulation of apoptosis and proliferation.
Yeung YS, Yip CW, Hon CC, Chow KY, Ma IC, Zeng F, Leung FC. Yeung YS, et al. Virology. 2008 Feb 5;371(1):32-43. doi: 10.1016/j.virol.2007.09.016. Epub 2007 Oct 24. Virology. 2008. PMID: 17961624 Free PMC article. - In vitro inhibition of feline coronavirus replication by small interfering RNAs.
McDonagh P, Sheehy PA, Norris JM. McDonagh P, et al. Vet Microbiol. 2011 Jun 2;150(3-4):220-9. doi: 10.1016/j.vetmic.2011.01.023. Epub 2011 Feb 1. Vet Microbiol. 2011. PMID: 21367541 Free PMC article. - Non-Coding RNAs and SARS-Related Coronaviruses.
Henzinger H, Barth DA, Klec C, Pichler M. Henzinger H, et al. Viruses. 2020 Dec 1;12(12):1374. doi: 10.3390/v12121374. Viruses. 2020. PMID: 33271762 Free PMC article. Review. - Prospects for RNAi Therapy of COVID-19.
Uludağ H, Parent K, Aliabadi HM, Haddadi A. Uludağ H, et al. Front Bioeng Biotechnol. 2020 Jul 30;8:916. doi: 10.3389/fbioe.2020.00916. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32850752 Free PMC article. Review.
References
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med., 348, (2003), 1986– 1994. - PubMed
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med., 348, (2003), 1977– 1985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous